The information on this page is intended for news media in the European Union only.
Latest News
July 22nd, 2024
Bloomington, Ind. — Cook Medical announced this morning it has signed a letter of intent with Astorg, a leading private equity firm with an extensive and successful track record in global healthcare investments, to purchase Cook’s Reproductive Health business (“Cook…
July 15th, 2024
 The 2024 Cook Medical Mini Marathon was launched today at the Strand Hotel. Now in its 26th year, up to 3,000 participants from all over Ireland are expected to take part. Cook Medical is proud to continue its sponsorship of…
The 2024 Cook Medical Mini Marathon was launched today at the Strand Hotel. Now in its 26th year, up to 3,000 participants from all over Ireland are expected to take part. Cook Medical is proud to continue its sponsorship of…
June 17th, 2024
 Cook Medical has released its Social Impact & Sustainability Report for 2023, highlighting the company’s commitment to working sustainably and responsibly across all of its businesses while fulfilling its purpose to improve the lives of everyone it serves. The report…
Cook Medical has released its Social Impact & Sustainability Report for 2023, highlighting the company’s commitment to working sustainably and responsibly across all of its businesses while fulfilling its purpose to improve the lives of everyone it serves. The report…
April 11th, 2024
 [caption id="attachment_2920" align="alignright" width="340"] Cook Medical's Resonance® Metallic Ureteral Stent [/caption] Bloomington, Ind. — The British Journal of Urology International Compass published a health economics model showing the benefits of Cook Medical’s Resonance® Metallic Ureteral Stent when used as a first-line…
[caption id="attachment_2920" align="alignright" width="340"] Cook Medical's Resonance® Metallic Ureteral Stent [/caption] Bloomington, Ind. — The British Journal of Urology International Compass published a health economics model showing the benefits of Cook Medical’s Resonance® Metallic Ureteral Stent when used as a first-line…
March 7th, 2024
 On 5 February, the Medicines and Healthcare products Regulatory Agency (MHRA) made a critical announcement: the agency updated their guidance on the use of paclitaxel-coated devices (PCDs). After carefully reviewing available evidence and receiving input from an independent expert working…
On 5 February, the Medicines and Healthcare products Regulatory Agency (MHRA) made a critical announcement: the agency updated their guidance on the use of paclitaxel-coated devices (PCDs). After carefully reviewing available evidence and receiving input from an independent expert working…
February 14th, 2024
The last several years have brought significant change to Cook. To ensure that we are positioned to be successful in this new world, our global leadership teams have implemented a new vision and strategic plan that were informed by extensive…
November 22nd, 2023
 Cook Medical has won Best Contribution to the Community Award at the Limerick Chamber Regional Business Awards. The award recognises organisations that have demonstrated exemplary commitment to social responsibility and made a positive impact on their local communities. The award…
Cook Medical has won Best Contribution to the Community Award at the Limerick Chamber Regional Business Awards. The award recognises organisations that have demonstrated exemplary commitment to social responsibility and made a positive impact on their local communities. The award…
November 1st, 2023
Bloomington, Indiana — In alignment with its 5-year strategic plan, Cook Medical announced today that CooperCompanies has acquired select products from Cook’s Maternal Fetal Medicine portfolio, as well as gynecological surgery products, and Doppler monitor technology. CooperCompanies (Nasdaq: COO), a…
October 9th, 2023
 Over 3,000 people took part in the Cook Medical Mini Marathon at the University of Limerick, on Sunday, 8 October. Now in its 25th year, the event saw participants of all ages and abilities run, jog, and walk the 5…
Over 3,000 people took part in the Cook Medical Mini Marathon at the University of Limerick, on Sunday, 8 October. Now in its 25th year, the event saw participants of all ages and abilities run, jog, and walk the 5…
September 27th, 2023
At the 2023 Vizient Connections Summit, Cook Medical won the Vizient Supply Assurance Supplier of the Year Award. This award demonstrates Cook’s transparency and commitment to communication during the supply chain process with healthcare organizations that are customers of Vizient,…
September 7th, 2023
 Limerick, Ireland — Cook Medical’s Advance Serenity® Hydrophilic PTA Balloon Dilation Catheter is now available with even more size options in Europe. In June, interventionalists who perform peripheral intervention procedures in the US and Canada got access to more sizes…
Limerick, Ireland — Cook Medical’s Advance Serenity® Hydrophilic PTA Balloon Dilation Catheter is now available with even more size options in Europe. In June, interventionalists who perform peripheral intervention procedures in the US and Canada got access to more sizes…
August 2nd, 2023
 Cook applauds the U.S. Food and Drug Administration’s recent update on paclitaxel-coated devices. We believe this is the best decision for physicians and patients. “We are grateful for the FDA’s latest update on paclitaxel. We applaud decisions that are based…
Cook applauds the U.S. Food and Drug Administration’s recent update on paclitaxel-coated devices. We believe this is the best decision for physicians and patients. “We are grateful for the FDA’s latest update on paclitaxel. We applaud decisions that are based…
July 17th, 2023
 The 2023 Cook Medical Mini Marathon was officially launched today at the Strand Hotel by Mayor of Limerick, Gerald Mitchell. Now in its 25th year, the race will return to the University of Limerick at 12pm on Sunday, October 8.…
The 2023 Cook Medical Mini Marathon was officially launched today at the Strand Hotel by Mayor of Limerick, Gerald Mitchell. Now in its 25th year, the race will return to the University of Limerick at 12pm on Sunday, October 8.…
June 26th, 2023
 Bloomington, Ind. — Following the publication of an animal study examining the performance of embolisation coils in arteries1 in the Journal of Vascular and Interventional Radiology (JVIR) in 2019, a second, similar study,2 published in the May 2023 issue of…
Bloomington, Ind. — Following the publication of an animal study examining the performance of embolisation coils in arteries1 in the Journal of Vascular and Interventional Radiology (JVIR) in 2019, a second, similar study,2 published in the May 2023 issue of…
June 7th, 2023
 The Hunt Museum, together with Cook Medical, is encouraging communities in Limerick to learn about the art of weaving willows through a new project that focuses on biodiversity and collaboration. The partnership is part of RECHARGE, a Horizon Europe funded…
The Hunt Museum, together with Cook Medical, is encouraging communities in Limerick to learn about the art of weaving willows through a new project that focuses on biodiversity and collaboration. The partnership is part of RECHARGE, a Horizon Europe funded…
May 15th, 2023
The email below, along with a video from our president and an FAQ document, was sent to all global Cook Medical employees on Monday, May 15 at 1pm EST. Memo header: Company announcement: A difficult step for our…
March 29th, 2023
 Novas Ireland and Cook Medical have launched a brand new art therapy studio on the grounds of Brother Russell House, Limerick. The purpose-built studio was the culmination of a partnership between Cook Medical and Novas, which saw Cook and its…
Novas Ireland and Cook Medical have launched a brand new art therapy studio on the grounds of Brother Russell House, Limerick. The purpose-built studio was the culmination of a partnership between Cook Medical and Novas, which saw Cook and its…
November 21st, 2022
 Cook Medical has won the Best Employer Award: Employee Value Proposition, at the Limerick Chamber Regional Business Awards, which were held at the Limerick Strand Hotel, on Friday November 18, 2022. The award recognises initiatives that support staff retention and…
Cook Medical has won the Best Employer Award: Employee Value Proposition, at the Limerick Chamber Regional Business Awards, which were held at the Limerick Strand Hotel, on Friday November 18, 2022. The award recognises initiatives that support staff retention and…
October 10th, 2022
For Cook, Corporate Social Responsibility is about more than just philanthropy or tracking environmental data. It's about using our core business opportunities, skills, and resources to not just do good business, but do good in the world. When people do…
September 26th, 2022
 Limerick, Ireland — Over 2,000 men and women from across Limerick and Ireland took part in the Cook Medical Mini Marathon today, Sunday, 25 September. Starting at University Limerick Sports Arena, participants of all ages and abilities came together to…
Limerick, Ireland — Over 2,000 men and women from across Limerick and Ireland took part in the Cook Medical Mini Marathon today, Sunday, 25 September. Starting at University Limerick Sports Arena, participants of all ages and abilities came together to…
August 8th, 2022
Limerick, Ireland – The 2022 Cook Medical Mini Marathon will return to the University of Limerick on Sunday 25th September for the first time since 2019. The race which is now in its 24th year was launched today, Monday, August 8, by…
June 2nd, 2022
 Limerick, Ireland — A study published in the Journal of Vascular and Interventional Radiology compared healthy porcine vessels treated with either a permanent polymer-coated drug-eluting stent (DES) or with Cook Medical’s polymer-free Zilver PTX DES.1 The study was performed by…
Limerick, Ireland — A study published in the Journal of Vascular and Interventional Radiology compared healthy porcine vessels treated with either a permanent polymer-coated drug-eluting stent (DES) or with Cook Medical’s polymer-free Zilver PTX DES.1 The study was performed by…
May 31st, 2022
 Limerick, Ireland — The MINC+™ Benchtop Incubator is now available to clinical embryologists and in-vitro fertilisation (IVF) clinics in select countries in the European Union. The MINC+ is the next generation of the MINC® Mini Incubator, a benchtop incubator that…
Limerick, Ireland — The MINC+™ Benchtop Incubator is now available to clinical embryologists and in-vitro fertilisation (IVF) clinics in select countries in the European Union. The MINC+ is the next generation of the MINC® Mini Incubator, a benchtop incubator that…
February 7th, 2022
 Limerick, Ireland — Cook Medical today announced it signed a letter of intent with CooperCompanies to sell the entirety of Cook’s Reproductive Health business within the MedSurg division. CooperCompanies (NYSE:COO), a publicly held healthcare company, is focused on women’s health and fertility solutions, providing…
Limerick, Ireland — Cook Medical today announced it signed a letter of intent with CooperCompanies to sell the entirety of Cook’s Reproductive Health business within the MedSurg division. CooperCompanies (NYSE:COO), a publicly held healthcare company, is focused on women’s health and fertility solutions, providing…
December 6th, 2021
 Cook Medical has been honoured with the Women in Leadership Company Initiative Award at the Irish MedTech Awards, which were held virtually on December 2, 2021. The award recognises companies that actively take steps in a dedicated initiative to increase…
Cook Medical has been honoured with the Women in Leadership Company Initiative Award at the Irish MedTech Awards, which were held virtually on December 2, 2021. The award recognises companies that actively take steps in a dedicated initiative to increase…
November 12th, 2021
Limerick, Ireland – Dr. Paul Gagne presented the three-year results of Cook Medical’s VIVO Clinical Study of the Zilver® Vena™ Venous Self-Expanding Stent at the Vascular Interventional Advances (VIVA) 2021 conference. The VIVO Clinical Study represents the first Investigational Device…
October 13th, 2021
 The 23rd Cook Medical Women’s Mini Marathon took place virtually from September 24–26. Over 1,500 participants, from all corners of Ireland and eight other countries, took part in their own time across the race weekend, while raising money for personally…
The 23rd Cook Medical Women’s Mini Marathon took place virtually from September 24–26. Over 1,500 participants, from all corners of Ireland and eight other countries, took part in their own time across the race weekend, while raising money for personally…
September 6th, 2021
 A commemorative tree planting ceremony has taken place on the grounds of Cook Medical to mark their 25th anniversary in Limerick. The ceremony was presided over by the mayor of Limerick, Councillor Daniel Butler. The company has planted 25 lime…
A commemorative tree planting ceremony has taken place on the grounds of Cook Medical to mark their 25th anniversary in Limerick. The ceremony was presided over by the mayor of Limerick, Councillor Daniel Butler. The company has planted 25 lime…
August 10th, 2021
 The 2021 Cook Medical Women’s Mini Marathon was launched today, Monday, August 9, by Limerick Mayor Daniel Butler at The Limerick Strand Hotel. Now in its 23rd year, the race will take place from September 24-26 as a virtual event, with…
The 2021 Cook Medical Women’s Mini Marathon was launched today, Monday, August 9, by Limerick Mayor Daniel Butler at The Limerick Strand Hotel. Now in its 23rd year, the race will take place from September 24-26 as a virtual event, with…
July 29th, 2021
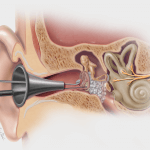 Bloomington, Ind. — Today, Cook Medical announced a new post-market study for the Biodesign® Otologic Repair Graft. Otologic repair on perforated eardrums usually requires physicians to harvest patient tissue from elsewhere in the body to repair the membrane. This Cook…
Bloomington, Ind. — Today, Cook Medical announced a new post-market study for the Biodesign® Otologic Repair Graft. Otologic repair on perforated eardrums usually requires physicians to harvest patient tissue from elsewhere in the body to repair the membrane. This Cook…
March 24th, 2021
 Cook Medical has been announced as the latest company to sign Business in the Community’s Low Carbon Pledge. Originally launched in 2018, the Low Carbon Pledge is the first dedicated pledge by Irish businesses to set industry standards on sustainability…
Cook Medical has been announced as the latest company to sign Business in the Community’s Low Carbon Pledge. Originally launched in 2018, the Low Carbon Pledge is the first dedicated pledge by Irish businesses to set industry standards on sustainability…
March 10th, 2021
Cook is a family company founded in 1963 and has grown into a global, multicultural organization. We were founded on core values of mutual respect, acting with integrity, and deeply committing to the quality of products and services we provide…
January 13th, 2021
Limerick, Ireland – The Litho EVO holmium laser is now available through Cook Medical in the United States, Austria, Germany, France, Ireland, Switzerland, and the United Kingdom. [caption id="attachment_7979" align="alignright" width="307"] The Quanta Litho EVO laser is now available through…
January 13th, 2021
Limerick, Ireland — Cook Medical today announced that the Blue Rhino G2-Multi Percutaneous Tracheostomy Introducer sets and trays are commercially available to physicians in select markets in Europe and the Middle East. This product is a new iteration of the…
November 19th, 2020
 Cook Medical's TriForce® Peripheral Crossing Set Limerick, Ireland. – Cook Medical’s TriForce® Peripheral Crossing Set is now available in Europe* to support procedures to treat patients with vascular obstructions. The TriForce Peripheral Crossing Set is designed to be percutaneously introduced…
Cook Medical's TriForce® Peripheral Crossing Set Limerick, Ireland. – Cook Medical’s TriForce® Peripheral Crossing Set is now available in Europe* to support procedures to treat patients with vascular obstructions. The TriForce Peripheral Crossing Set is designed to be percutaneously introduced…
November 15th, 2020
 Bloomington, Ind. – At this year’s Vascular Interventional Advances (VIVA) conference, Dr. Anthony Comerota presented data on the Zilver® Vena™ Venous Self-Expanding Stent that demonstrates durable patency and symptom relief in patients over an extended period of time.1 Zilver Vena is…
Bloomington, Ind. – At this year’s Vascular Interventional Advances (VIVA) conference, Dr. Anthony Comerota presented data on the Zilver® Vena™ Venous Self-Expanding Stent that demonstrates durable patency and symptom relief in patients over an extended period of time.1 Zilver Vena is…
September 12th, 2020
 Bloomington, Ind. – At this year’s Cardiovascular and Interventional Radiological Society of Europe (CIRSE) conference, Michael D. Dake, MD, presented a prediction model for treatment outcomes for patients treated with Zilver PTX. The model includes data generated from more than…
Bloomington, Ind. – At this year’s Cardiovascular and Interventional Radiological Society of Europe (CIRSE) conference, Michael D. Dake, MD, presented a prediction model for treatment outcomes for patients treated with Zilver PTX. The model includes data generated from more than…
September 1st, 2020
 Bloomington, Ind. – A study recently published in BMC Urology compared Cook Medical’s Black Silicone Filiform Double Pigtail Ureteral Stent Set to a traditional polyurethane ureteral stent and found that on two separate patient surveys, the black silicone stent was…
Bloomington, Ind. – A study recently published in BMC Urology compared Cook Medical’s Black Silicone Filiform Double Pigtail Ureteral Stent Set to a traditional polyurethane ureteral stent and found that on two separate patient surveys, the black silicone stent was…
August 18th, 2020
 This year marks the first time that the Cook Medical Women’s Mini marathon will take place as a virtual event. 100% of proceeds will go to local and national charities. 2020 marks fifth consecutive year of Cook Medical’s title sponsorship,…
This year marks the first time that the Cook Medical Women’s Mini marathon will take place as a virtual event. 100% of proceeds will go to local and national charities. 2020 marks fifth consecutive year of Cook Medical’s title sponsorship,…
July 2nd, 2020
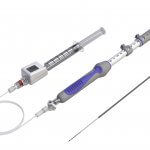 Limerick, Ireland– Cook Medical recently received a CE mark to market a new device, the EchoTip® Insight™ Portosystemic Pressure Gradient, in the European Union. The EchoTip Insight is an endoscopic ultrasound device that allows endoscopists to measure portal pressure. Cook…
Limerick, Ireland– Cook Medical recently received a CE mark to market a new device, the EchoTip® Insight™ Portosystemic Pressure Gradient, in the European Union. The EchoTip Insight is an endoscopic ultrasound device that allows endoscopists to measure portal pressure. Cook…
June 30th, 2020
The Indianapolis Business Journal highlighted Cook Medical and Cook Group as one of the top 50 privately owned companies in Indiana. Cook Group ranked third in the state of Indiana this year. Reporter Sam Stall interviewed several Cook executives about how we keep our…
June 15th, 2020
This is a painful time in our country. It’s unfathomable that tragedies such as the deaths of George Floyd, Breonna Taylor, and Ahmaud Arbery continue to happen. Sadly, as people of color know all too well, they do happen every…
May 1st, 2020
First, we want to thank everyone on the front lines fighting this pandemic. We acknowledge the sacrifices you are making to protect your own families while meeting your obligations to patients. Across the global healthcare industry, the COVID-19 pandemic has…
January 29th, 2020
Bloomington, Ind. – Cook Medical and Bentley today announced that they are collaborating in clinical trials involving Cook’s fenestrated and branch stent grafts and Bentley’s covered stents. About Cook Medical Since 1963, Cook Medical has worked closely with physicians to develop technologies…
January 17th, 2020
 Bloomington, Ind. — Cook Medical’s innovative Hemospray® product received the 2019 Healio Disruptive Innovators Award in the Industry Breakthrough category. Healio, a healthcare news and education organisation, presented the awards on 27 October at the American College of Gastroenterology Annual Scientific…
Bloomington, Ind. — Cook Medical’s innovative Hemospray® product received the 2019 Healio Disruptive Innovators Award in the Industry Breakthrough category. Healio, a healthcare news and education organisation, presented the awards on 27 October at the American College of Gastroenterology Annual Scientific…
January 10th, 2020
 The limited take-up of Leaving Certificate engineering among girls is largely due to a lack of access to the subject, according to global medtech company Cook Medical. Recent figures from the Department of Education show the interest in STEM subjects…
The limited take-up of Leaving Certificate engineering among girls is largely due to a lack of access to the subject, according to global medtech company Cook Medical. Recent figures from the Department of Education show the interest in STEM subjects…
September 29th, 2019
 Almost 3,000 women from across Limerick and Ireland took part in the Cook Medical Women’s Mini Marathon today, Sunday, 29 September. Starting at University Limerick Sports Arena, participants of all ages and abilities came together to run, jog and walk…
Almost 3,000 women from across Limerick and Ireland took part in the Cook Medical Women’s Mini Marathon today, Sunday, 29 September. Starting at University Limerick Sports Arena, participants of all ages and abilities came together to run, jog and walk…
September 27th, 2019
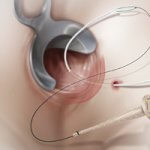 Limerick, Ireland — The National Institute for Health and Care Excellence (NICE) Interventional Procedures Advisory Committee in the UK has recommended bioprosthetic fistula plugs as a treatment option for anal fistulas. [caption id="attachment_2202" align="alignright" width="300"] Cook Medical’s unique Biodesign® Anal Fistula…
Limerick, Ireland — The National Institute for Health and Care Excellence (NICE) Interventional Procedures Advisory Committee in the UK has recommended bioprosthetic fistula plugs as a treatment option for anal fistulas. [caption id="attachment_2202" align="alignright" width="300"] Cook Medical’s unique Biodesign® Anal Fistula…
September 10th, 2019
 Barcelona, Spain — Michael D. Dake, MD, presented at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) conference regarding new data on the use of Zilver® PTX®, Cook Medical’s paclitaxel-coated stent for peripheral arterial disease (PAD). Dr Dake is the…
Barcelona, Spain — Michael D. Dake, MD, presented at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) conference regarding new data on the use of Zilver® PTX®, Cook Medical’s paclitaxel-coated stent for peripheral arterial disease (PAD). Dr Dake is the…
August 30th, 2019
July 16th, 2019
 The 2019 Cook Medical Women’s Mini Marathon was launched by Limerick Mayor Michael Sheahan at The Strand Hotel. Now in its 21st year, up to 3,000 participants from all over Ireland are expected to take part. The event kicks off at 12:00…
The 2019 Cook Medical Women’s Mini Marathon was launched by Limerick Mayor Michael Sheahan at The Strand Hotel. Now in its 21st year, up to 3,000 participants from all over Ireland are expected to take part. The event kicks off at 12:00…
June 24th, 2019
Limerick, Ireland — A newly published prospective, randomised controlled trial concluded that 20 gauge fine needle biopsy (FNB) needles consistently outperformed 25 gauge fine needle aspiration (FNA) needles in terms of histological yield and diagnostic accuracy in pancreatic and non-pancreatic…
Cook Medical calls for continued investment to support innovation in the medtech industry in Ireland
June 11th, 2019
 Development of innovative minimally invasive devices, personalised healthcare, the connected health model and a more streamlined approach to clinical trials could deliver savings for the Irish healthcare system, reduce patient recovery time and improve patients’ lives, according to Cook Medical.…
Development of innovative minimally invasive devices, personalised healthcare, the connected health model and a more streamlined approach to clinical trials could deliver savings for the Irish healthcare system, reduce patient recovery time and improve patients’ lives, according to Cook Medical.…
May 20th, 2019
Bloomington, Ind. — Following the recent FDA approval of Cook Medical’s Zenith Dissection Endovascular System, Cooper University Health Care in Camden, New Jersey, has treated the first patient in the U.S. with the device as part of Cook’s U.S. commercial…
May 13th, 2019
 The Irish Examiner featured an interview about Cook Medical and our dedication to improving patient treatment worldwide. Pat Burke spoke with journalist Joe Dermody about his new role as vice president and general manager of Cook Medical's Irish manufacturing facility and about…
The Irish Examiner featured an interview about Cook Medical and our dedication to improving patient treatment worldwide. Pat Burke spoke with journalist Joe Dermody about his new role as vice president and general manager of Cook Medical's Irish manufacturing facility and about…
April 18th, 2019
Limerick, Ireland — This week, Cook Medical made patient-level data from the Zilver® PTX® randomised control trial (RCT) available by request on cookmedical.com. Cook provides this data to encourage further collaboration with researchers to benefit patients with peripheral arterial disease. “We will…
April 3rd, 2019
 Cook Medical has announced Pat Burke as the new vice president and general manager of its Ireland manufacturing facility, based in the National Technology Park in Limerick. In this role, Burke will oversee the day-to-day running of Cook Medical’s manufacturing…
Cook Medical has announced Pat Burke as the new vice president and general manager of its Ireland manufacturing facility, based in the National Technology Park in Limerick. In this role, Burke will oversee the day-to-day running of Cook Medical’s manufacturing…
March 25th, 2019
 A new animal study concludes that fewer fibred embolisation coils are needed to achieve acute occlusion when compared with similar bare metal coils. The study was presented today at an industry-sponsored symposium during the 2019 annual meeting of the Society…
A new animal study concludes that fewer fibred embolisation coils are needed to achieve acute occlusion when compared with similar bare metal coils. The study was presented today at an industry-sponsored symposium during the 2019 annual meeting of the Society…
March 6th, 2019
 [caption id="attachment_2087" align="alignright" width="300"] Cook Medical engineers with students from Monaleen NS[/caption] To mark Engineers Week 2019, global medical devices company Cook Medical is educating students in local schools on medtech engineering by using augmented reality (AR) technology. Starting with…
[caption id="attachment_2087" align="alignright" width="300"] Cook Medical engineers with students from Monaleen NS[/caption] To mark Engineers Week 2019, global medical devices company Cook Medical is educating students in local schools on medtech engineering by using augmented reality (AR) technology. Starting with…
September 30th, 2018
 [caption id="attachment_2078" align="alignright" width="300"] Angela Moloney of Cook Medical at the start line[/caption] Almost 2,800 runners, joggers and walkers participated in the Cook Medical Women’s Mini Marathon today, Sunday 30 September raising thousands of euro for local and national charities and…
[caption id="attachment_2078" align="alignright" width="300"] Angela Moloney of Cook Medical at the start line[/caption] Almost 2,800 runners, joggers and walkers participated in the Cook Medical Women’s Mini Marathon today, Sunday 30 September raising thousands of euro for local and national charities and…
July 30th, 2018
The ongoing lack of an agreement between London and Brussels is a challenge for companies that serve patients both in the UK and the EU. Cook Medical, which employs more than 850 people in Limerick, has today called on the…
June 7th, 2018
 [caption id="attachment_2056" align="alignright" width="300"] Pictured are Darach McGrath, Director of Global Research and Development, Cook Medical, Laura Ryan, Head of Communications for Limerick City and County Council; Bill Doherty, Executive Vice President Cook Medical EMEA; Orlaith Borthwick, Programme Manager at…
[caption id="attachment_2056" align="alignright" width="300"] Pictured are Darach McGrath, Director of Global Research and Development, Cook Medical, Laura Ryan, Head of Communications for Limerick City and County Council; Bill Doherty, Executive Vice President Cook Medical EMEA; Orlaith Borthwick, Programme Manager at…
May 18th, 2018
 Bloomington, Ind. – Last week, Dave Reed was recognised as a leader in healthcare operations by the Global Healthcare Exchange (GHX). The Supply Chain Leadership award recognises companies and individuals who make a difference in healthcare, transforming supply chain automation,…
Bloomington, Ind. – Last week, Dave Reed was recognised as a leader in healthcare operations by the Global Healthcare Exchange (GHX). The Supply Chain Leadership award recognises companies and individuals who make a difference in healthcare, transforming supply chain automation,…
May 4th, 2018
Bloomington, Ind. – Cook Medical has announced Ross Harvey as the new director of global Customer Support & Delivery (CSD). In this role, Harvey will oversee the global customer service teams, global distribution centres and warehouses. These teams will work…
April 20th, 2018
Bloomington, Ind. – Today, Christina Anné was named vice president of Distribution Channel Management (DCM) for Cook Medical. The DCM team will be dedicated to serving the needs of Cook’s distributors around the world and to ensure compliant and ethical…
March 7th, 2018
Bloomington, Ind. – Today, Cook Medical announced Dan Kaiser as the leader of global research and development (R&D). This dedicated engineering team will connect product development more closely to customer needs and accelerate the process of getting new products to market. “Our…
February 28th, 2018
Bloomington, Ind. – Today, Cook Medical announced the leaders of its two new business divisions. Mark Breedlove has been named vice president of Cook’s Vascular division and DJ Sirota has been named vice president of Cook’s MedSurg division. The new…
February 20th, 2018
 Bloomington, Ind. – Today, Cook Medical announced key changes that simplify its organisational structure to better support customers. These changes realign the current sales, marketing, research and development, and customer service teams in addition to establishing new distribution channel management…
Bloomington, Ind. – Today, Cook Medical announced key changes that simplify its organisational structure to better support customers. These changes realign the current sales, marketing, research and development, and customer service teams in addition to establishing new distribution channel management…
January 11th, 2018
When it comes to the world of STEM (Science, Technology, Engineering and Maths), a solid grasp of numbers and logic is essential. But it’s not everything, commented Darach McGrath, Director of Engineering of Cook Medical. The Limerick-based medical device company…
November 1st, 2017
 More than 2,400 women took part in the Cook Medical Limerick Women's Mini Marathon today (Sunday) to benefit a variety of charities including Sophie’s Journey, Crumlin Children’s Hospital through fundraising by race participants. The 5km and 10km courses took runners,…
More than 2,400 women took part in the Cook Medical Limerick Women's Mini Marathon today (Sunday) to benefit a variety of charities including Sophie’s Journey, Crumlin Children’s Hospital through fundraising by race participants. The 5km and 10km courses took runners,…
October 25th, 2017
Limerick, Ireland – Cook Medical is pleased to announce that the 25th UEG Week 2017 in Barcelona will be the stage for the European launch of the next generation Acrobat 2 Calibrated Tip Wire Guide. Incorporating technology from Cook Interventional…
September 11th, 2017
 Funds raised will be used to support local and regional charities and community groups 5km and 10km courses will bring walkers, joggers and runners around the grounds of the University of Limerick and beyond. Today, Alice O’Dwyer, VP of HR…
Funds raised will be used to support local and regional charities and community groups 5km and 10km courses will bring walkers, joggers and runners around the grounds of the University of Limerick and beyond. Today, Alice O’Dwyer, VP of HR…
September 5th, 2017
Limerick, Ireland: Cook Medical’s Urology business announced that it will begin distributing the Cellvizio® Confocal Laser Endomicroscopy (CLE) System manufactured by Mauna Kea Technologies. With the Cellvizio system, a physician can visualise the internal microstructure of tissues in real time…
June 21st, 2017
 Cook Medical senior engineers visit Castletroy Gaelscoil to talk about careers in Ireland’s medical technology sector ahead of MedTech Week, 19-23 June 2017 Speaking today, Alice O’Dwyer, Vice President of HR at Cook Medical in Limerick says: “Collaboration between…
Cook Medical senior engineers visit Castletroy Gaelscoil to talk about careers in Ireland’s medical technology sector ahead of MedTech Week, 19-23 June 2017 Speaking today, Alice O’Dwyer, Vice President of HR at Cook Medical in Limerick says: “Collaboration between…
March 22nd, 2017
 [caption id="attachment_1916" align="alignright" width="300"] The KUBE team outside of Cook Medical in Limerick[/caption] Cook Medical, a leading MedTech company based in Limerick, is hosting ‘The KUBE’ charity event in aid of the Mid-West Cancer Foundation on Saturday, 1 April at…
[caption id="attachment_1916" align="alignright" width="300"] The KUBE team outside of Cook Medical in Limerick[/caption] Cook Medical, a leading MedTech company based in Limerick, is hosting ‘The KUBE’ charity event in aid of the Mid-West Cancer Foundation on Saturday, 1 April at…
February 23rd, 2017
 [caption id="attachment_1911" align="alignright" width="240"] Universa® Percutaneous Drainage Catheters[/caption] Limerick, Ireland — Cook Medical has rounded out the Universa line with the introduction of five new sets for percutaneous urinary drainage. Each set includes a catheter and accessories for specific procedures.…
[caption id="attachment_1911" align="alignright" width="240"] Universa® Percutaneous Drainage Catheters[/caption] Limerick, Ireland — Cook Medical has rounded out the Universa line with the introduction of five new sets for percutaneous urinary drainage. Each set includes a catheter and accessories for specific procedures.…
January 13th, 2017
Cook Medical, the global medical technology company that employs 850 people in Ireland is encouraging industry, government and educational bodies to collaborate in promoting engineering education for primary and secondary pupils. The company is at the Ibec stand hosted by…
December 19th, 2016
[caption id="attachment_1890" align="alignright" width="300"] Cook Medical's European Distribution Centre in Baesweiler, Germany[/caption] Baesweiler, Germany: Cook Medical’s European Distribution Centre (EUDC) located in Baesweiler, Germany, is celebrating its fifth birthday this December. The EUDC is the storage and distribution centre for…
November 1st, 2016
 Limerick, Ireland: Over 2,500 athletes participated in the Cook Medical Women’s Mini Marathon this weekend. Now in its 18th year, the event raises funds for worthy local and national causes, with over 75 charities set to benefit from this year’s…
Limerick, Ireland: Over 2,500 athletes participated in the Cook Medical Women’s Mini Marathon this weekend. Now in its 18th year, the event raises funds for worthy local and national causes, with over 75 charities set to benefit from this year’s…
September 22nd, 2016
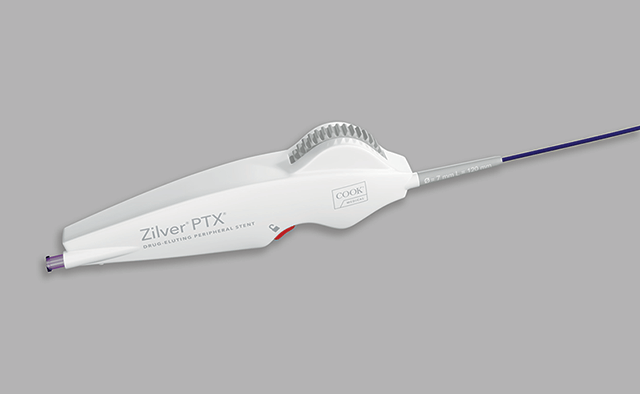 Limerick, Ireland— Cook Medical launches Zilver® PTX® drug-eluting peripheral stent thumbwheel delivery system in France. The French Health Ministry has now approved reimbursement for the Zilver PTX thumbwheel delivery system. The rotating thumbwheel system, which is available for purchase in…
Limerick, Ireland— Cook Medical launches Zilver® PTX® drug-eluting peripheral stent thumbwheel delivery system in France. The French Health Ministry has now approved reimbursement for the Zilver PTX thumbwheel delivery system. The rotating thumbwheel system, which is available for purchase in…
August 16th, 2016
MedTech company encourages school guidance counsellors to work with industry to promote work placement programmes which give students an insight into working in careers relating to Science, Technology, Engineer and Maths (STEM) Mid-West Career Guidance Counsellors visit Cook Medical as…
August 10th, 2016
 Cook Group and Cook Medical sat down with The Herald-Times, the local newspaper in Bloomington, Indiana, to discuss Cook's transformation and preparation for healthcare of the future. These interviews resulted in a four-day series by veteran reporter Bill Strother as described…
Cook Group and Cook Medical sat down with The Herald-Times, the local newspaper in Bloomington, Indiana, to discuss Cook's transformation and preparation for healthcare of the future. These interviews resulted in a four-day series by veteran reporter Bill Strother as described…
August 9th, 2016
 Limerick, Irl: Limerick Women’s Mini Marathon has announced that their title sponsor for the next three years will be Castletroy based company, Cook Medical. The 19th Women’s Mini Marathon will be held on Sunday 30th October and hopes to attract…
Limerick, Irl: Limerick Women’s Mini Marathon has announced that their title sponsor for the next three years will be Castletroy based company, Cook Medical. The 19th Women’s Mini Marathon will be held on Sunday 30th October and hopes to attract…
July 18th, 2016
MedTech worth €12.6 billion to economy each year, but new advancements demand up-to-date skills Cook Medical made the comments at MedTech Week, a pan-European initiative to raise awareness of medical technology Limerick, Irl: Ireland will remain a global medtech leader…
May 16th, 2016
Cook Medical has initiated a global, voluntary recall of all catheters with Beacon® Tip technology. This recall includes all lots of catheters with the Beacon Tip technology. The catheters were recalled in the U.S. on April 15, 2016 and April 21,…
March 2nd, 2016
 Limerick, Ireland – In 2016 Cook Medical will celebrate 20 years of operations in the National Technology Park where it has grown from a primary team of less than a dozen people to a staff of over 800, who are…
Limerick, Ireland – In 2016 Cook Medical will celebrate 20 years of operations in the National Technology Park where it has grown from a primary team of less than a dozen people to a staff of over 800, who are…
March 1st, 2016
 Bloomington, Ind. – Cook Group is pleased to announce the promotion of Barry Slowey to president of Cook Endoscopy/Cook Winston-Salem. As president, Slowey will oversee the various functions in Winston-Salem, North Carolina, and will maintain his leadership responsibilities for the…
Bloomington, Ind. – Cook Group is pleased to announce the promotion of Barry Slowey to president of Cook Endoscopy/Cook Winston-Salem. As president, Slowey will oversee the various functions in Winston-Salem, North Carolina, and will maintain his leadership responsibilities for the…
December 2nd, 2015
Limerick, Ireland – A recent study by Breda et al shows that the Flexor® Parallel™ Rapid Release™ Ureteral Access Sheath (UAS) provides physicians an option to use one wire guide for treatment and diagnostic purposes during flexible ureteroscopy (fURS) procedures.…
November 17th, 2015
 Limerick, Irl. – Cook Medical’s Endoscopy clinical division announces the introduction of the latest addition to the EchoTip ProCore® product line, the EchoTip ProCore 20 gage needle with ReCoil Stylet™. This needle’s flexible design allows clinicians to obtain histological samples from…
Limerick, Irl. – Cook Medical’s Endoscopy clinical division announces the introduction of the latest addition to the EchoTip ProCore® product line, the EchoTip ProCore 20 gage needle with ReCoil Stylet™. This needle’s flexible design allows clinicians to obtain histological samples from…
October 12th, 2015
Our hearts are heavy as we mourn the passing of our friend and coworker. On Friday night, Bill Gibbons, our vice president of engineering, and his daughter were involved in a plane crash in eastern Tennessee. Bill was 45 and…
September 29th, 2015
Lisbon, Portugal – Dr Gerard O’Sullivan announced preliminary results from a European study of venous stents today at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) 2015, a European meeting for interventional radiologists and vascular specialists. Dr O’Sullivan is the…
September 28th, 2015
Bloomington, Ind. — Dr. Kimihiko Kichikawa, Department of Radiology at Nara Medical University in Japan, reported two-year results of the Zilver® PTX® post-market surveillance (PMS) study on September 27, 2015, in Lisbon, Portugal. Dr. Kichikawa presented initial target data on…
June 17th, 2015
Bloomington, Ind. — Cook Group announced today that Kem Hawkins, president of Cook Group Incorporated, will retire on July 1 after 34 years with the Cook organisation. Hawkins, who has been transitioning his role in the company over the past…
May 22nd, 2015
 Bloomington, Ind., May 22 2015 — Cook Medical is pleased to be one of the global medical device companies to collaborate internationally with regulatory authorities on the Medical Device Single Audit Program (MDSAP). The value of developing a global program…
Bloomington, Ind., May 22 2015 — Cook Medical is pleased to be one of the global medical device companies to collaborate internationally with regulatory authorities on the Medical Device Single Audit Program (MDSAP). The value of developing a global program…
March 16th, 2015
 Limerick, Ireland — CE Mark has been awarded to the new rotating thumbwheel deployment system for Cook Medical’s Zilver® PTX® drug-eluting peripheral stent. The new system, which is now available for purchase in the UK, Ireland, Germany, Spain and Italy,…
Limerick, Ireland — CE Mark has been awarded to the new rotating thumbwheel deployment system for Cook Medical’s Zilver® PTX® drug-eluting peripheral stent. The new system, which is now available for purchase in the UK, Ireland, Germany, Spain and Italy,…
March 2nd, 2015
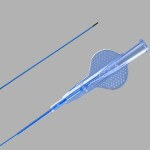 Limerick, Ireland, February 25, 2015. – Clinicians who treat obstructive salivary gland disorders now have the option of a specialised catheter designed to irrigate the salivary duct and flush out stone fragments. Cook Medical’s SialoCath™ Salivary Duct Catheter is a flexible,…
Limerick, Ireland, February 25, 2015. – Clinicians who treat obstructive salivary gland disorders now have the option of a specialised catheter designed to irrigate the salivary duct and flush out stone fragments. Cook Medical’s SialoCath™ Salivary Duct Catheter is a flexible,…
February 9th, 2015
 Limerick, Ireland, February 4, 2015 – Historic five-year results from the world’s largest clinical trial of a drug-eluting stent for treating peripheral arterial disease (PAD) were presented last week at the Leipzig Interventional Course, in Germany. The data confirm long-term patency…
Limerick, Ireland, February 4, 2015 – Historic five-year results from the world’s largest clinical trial of a drug-eluting stent for treating peripheral arterial disease (PAD) were presented last week at the Leipzig Interventional Course, in Germany. The data confirm long-term patency…
November 4th, 2014
 Las Vegas, Nev., November 4, 2014 — Five-year results from the largest and longest-running clinical trial of a drug-eluting stent for treating peripheral arterial disease (PAD) confirmed long-term patency for patients treated with Zilver PTX. The results were presented today by…
Las Vegas, Nev., November 4, 2014 — Five-year results from the largest and longest-running clinical trial of a drug-eluting stent for treating peripheral arterial disease (PAD) confirmed long-term patency for patients treated with Zilver PTX. The results were presented today by…
September 24th, 2014
 Bloomington, Ind., September 24, 2014 – Cook Medical is pleased to announce that Jean-Marc Creissel has been named vice president of the Urology clinical division. He has served as global leader of the business unit since 2010. As vice president, Creissel will…
Bloomington, Ind., September 24, 2014 – Cook Medical is pleased to announce that Jean-Marc Creissel has been named vice president of the Urology clinical division. He has served as global leader of the business unit since 2010. As vice president, Creissel will…
May 29th, 2014
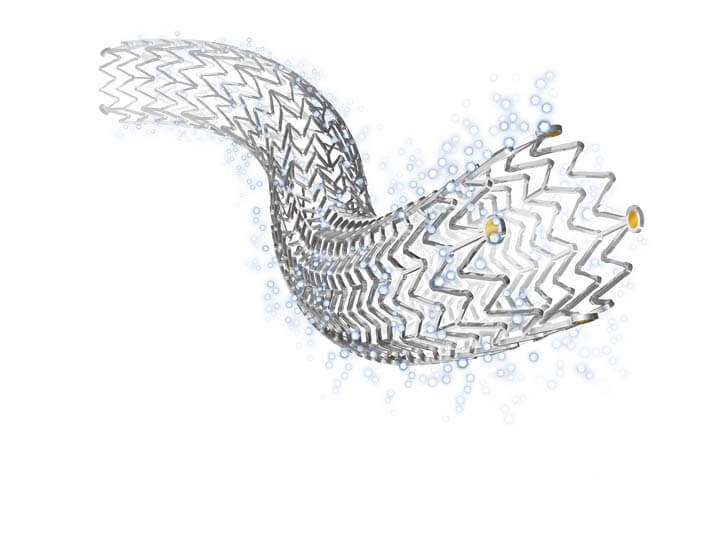 Bloomington, Ind. — One-year follow-up data from 907 Japanese patients who received the Zilver® PTX® drug-eluting stent showed positive results in keeping open the superficial femoral artery (SFA). The postmarket surveillance (PMS) study, designed to evaluate the stent’s performance in real-world patient use,…
Bloomington, Ind. — One-year follow-up data from 907 Japanese patients who received the Zilver® PTX® drug-eluting stent showed positive results in keeping open the superficial femoral artery (SFA). The postmarket surveillance (PMS) study, designed to evaluate the stent’s performance in real-world patient use,…
May 6th, 2014
San Francisco, Calif. — A quarter century ago, doctors treating patients with implanted cardiac pacemakers had a big problem. Their patients were outliving the complex electrical devices that gave them an acceptable quality of life. Pacemaker lead wires that deliver electricity…
April 23rd, 2014
 Bloomington, Ind., April 21, 2014 – The Instinct® Endoscopic Clip is now available to gastroenterologists in major global markets. The clip is used to stop gastrointestinal (GI) tract bleeding, which is a condition that can be challenging to treat because of the…
Bloomington, Ind., April 21, 2014 – The Instinct® Endoscopic Clip is now available to gastroenterologists in major global markets. The clip is used to stop gastrointestinal (GI) tract bleeding, which is a condition that can be challenging to treat because of the…
April 11th, 2014
Bloomington, Ind. – Cook Medical today announced the introduction of its 2.9 Fr size Cantata® microcatheter with a .027 inch inner diameter (ID) and the Alight™ microwire. This .027 ID Cantata micro-catheter complements the existing .021 and .025 inch ID microcatheters, completing Cook's…
April 8th, 2014
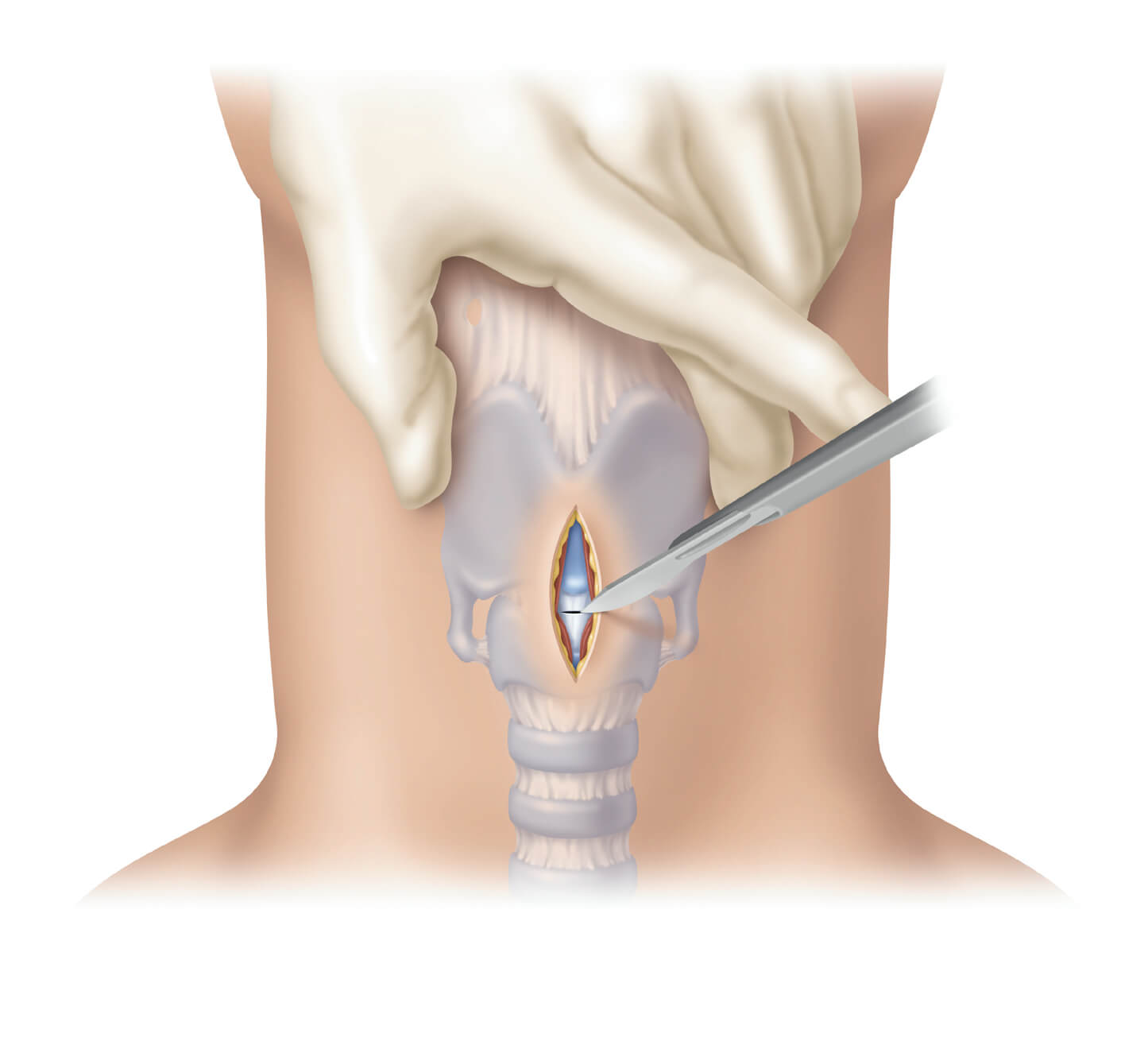 International Symposium on Intensive Care and Emergency Medicine, Brussels, Belgium, 18 March, 2014 — In response to the NAP4 study addressing the clinical need for both Seldinger* and surgical cricothyrotomy procedures to be taught side-by-side[1], Cook Medical today announces a…
International Symposium on Intensive Care and Emergency Medicine, Brussels, Belgium, 18 March, 2014 — In response to the NAP4 study addressing the clinical need for both Seldinger* and surgical cricothyrotomy procedures to be taught side-by-side[1], Cook Medical today announces a…
February 19th, 2014
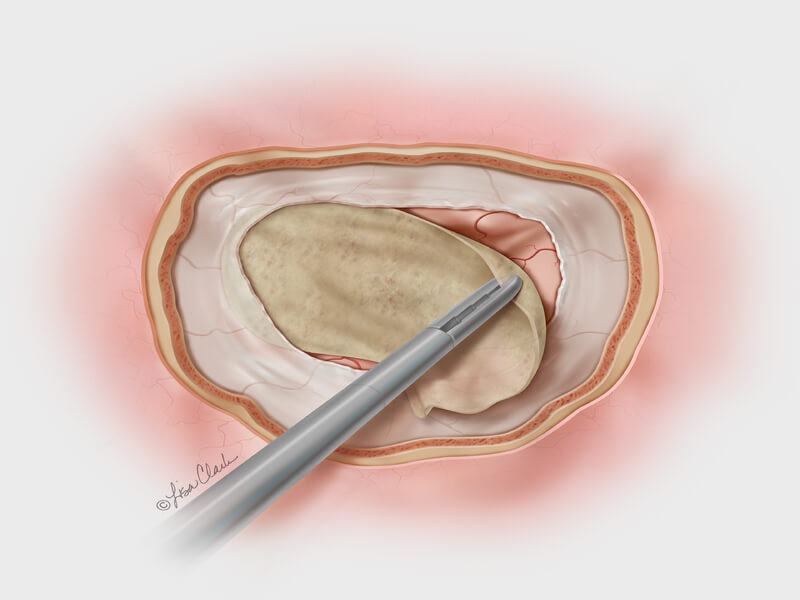 Bloomington, Ind. – Cook Medical has launched a new treatment option for otolaryngologists (ear, nose, and throat specialists) who repair the dura mater following cerebrospinal fluid (CSF) leaks at the base of the skull. Cook’s Biodesign® Duraplasty Graft is the…
Bloomington, Ind. – Cook Medical has launched a new treatment option for otolaryngologists (ear, nose, and throat specialists) who repair the dura mater following cerebrospinal fluid (CSF) leaks at the base of the skull. Cook’s Biodesign® Duraplasty Graft is the…
February 12th, 2014
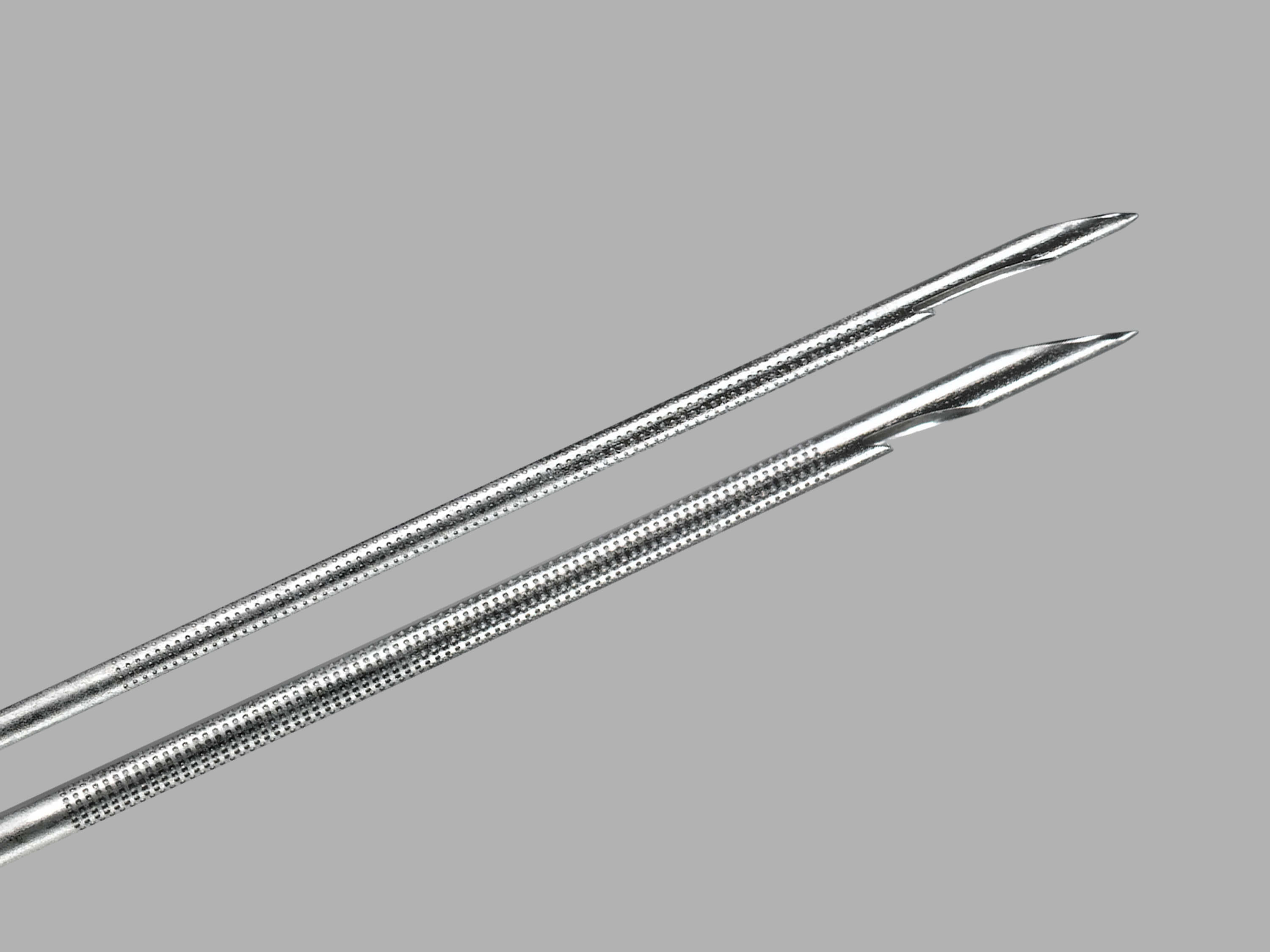 Bloomington, Ind.—Cook Medical is introducing the first endobronchial ultrasound (EBUS) needle in the U.S. and Europe that can acquire histological samples. The EchoTip® ProCore™ Endobronchial Ultrasound Needle gives physicians the ability to retrieve both cell and tissue samples from lymph…
Bloomington, Ind.—Cook Medical is introducing the first endobronchial ultrasound (EBUS) needle in the U.S. and Europe that can acquire histological samples. The EchoTip® ProCore™ Endobronchial Ultrasound Needle gives physicians the ability to retrieve both cell and tissue samples from lymph…
December 4th, 2013
 Bloomington, Ind. – Mark Breedlove, a 19-year veteran of Cook Medical, has been named global leader for the company’s Peripheral Interventional (PI) division. 'As our largest division, PI plays a vital role in Cook Medical’s global success,' said Pete Yonkman, president…
Bloomington, Ind. – Mark Breedlove, a 19-year veteran of Cook Medical, has been named global leader for the company’s Peripheral Interventional (PI) division. 'As our largest division, PI plays a vital role in Cook Medical’s global success,' said Pete Yonkman, president…
December 4th, 2013
 Bloomington, Ind. – Rob Lyles, a veteran leader of Cook Medical’s Peripheral Intervention (PI) division, has been promoted to executive vice president in a move to further invigorate Cook’s drive toward new, cutting edge technologies and patient therapies. He will transition…
Bloomington, Ind. – Rob Lyles, a veteran leader of Cook Medical’s Peripheral Intervention (PI) division, has been promoted to executive vice president in a move to further invigorate Cook’s drive toward new, cutting edge technologies and patient therapies. He will transition…
November 13th, 2013
 Bloomington, Ind. – Kem Hawkins, president of Cook Group Incorporated, announced today that Pete Yonkman will lead Cook Group’s medical companies as president of Cook Medical. Hawkins will continue serving as president of Cook Group. 'Cook is an amazing company run…
Bloomington, Ind. – Kem Hawkins, president of Cook Group Incorporated, announced today that Pete Yonkman will lead Cook Group’s medical companies as president of Cook Medical. Hawkins will continue serving as president of Cook Group. 'Cook is an amazing company run…
November 13th, 2013
 Bloomington, Ind. — Cook Medical has today officially opened its new state-of-the-art research and development (R&D) Innovation Centre at its plant in Limerick, Ireland. [brightcove 2955909007001] The Innovation Centre together, with the expansion of cleanrooms, packaging, storage and other facilities, represents…
Bloomington, Ind. — Cook Medical has today officially opened its new state-of-the-art research and development (R&D) Innovation Centre at its plant in Limerick, Ireland. [brightcove 2955909007001] The Innovation Centre together, with the expansion of cleanrooms, packaging, storage and other facilities, represents…
October 8th, 2013
Las Vegas, Nev. — Four-year data from the Zilver® PTX® Randomized Controlled Trial of Paclitaxel-Eluting Stents for Femoropopliteal Disease from Cook Medical presented today at the 2013 Vascular Interventional Advances (VIVA) meeting demonstrates 75 per cent primary patency in the superficial…
October 2nd, 2013
Bloomington, Ind.—Cook Medical today marks the one-year anniversary of the formal launch of its Otolaryngology - Head and Neck Surgery Division (OHNS). Since launching at the American Academy of Otolaryngology - Head and Neck Surgery (AAO-HNS) conference in 2012, the division has…
September 5th, 2013
Bloomington, Ind. —Cook Medical has a new device to simplify percutaneous nephrolithotomy (PCNL) procedures, during which physicians break up and remove large kidney stones, or can use it in the bladder to break up large bladder stones. LithAssist™ combines suction…
August 22nd, 2013
Bloomington, Ind. – Nicky James, a 15-year veteran of Cook Medical, has been named vice president and global leader of Cook’s Aortic Intervention (AI) clinical division. Phil Nowell, who has held that position for five years, will return to his native…
August 8th, 2013
Bloomington, Ind. — Cook Medical has completed patient enrolment in a study evaluating a technique for achieving vascular access via below-the-knee arteries. The new access technique could be used in treating peripheral arterial disease (PAD), including patients with critical limb ischemia…
August 7th, 2013
Bloomington, Ind. – Cook Medical is again shipping its Zilver® PTX® Drug-Eluting Peripheral Stent to medical centers in the U.S., Japan, Europe and other major markets. The shipments follow a brief period of unavailability due to a voluntary recall by Cook…
June 19th, 2013
Bloomington, Ind. — Cook Medical is making its Hercules® 3 Stage Wire Guided Oesophageal Balloon available to otolaryngologists to treat patients with gastrointestinal (GI) strictures. The device will be offered through Cook’s Otolaryngology-Head and Neck Surgery (OHNS) clinical division, while also…
June 7th, 2013
Bloomington, Ind. – In an effort to provide surgeons and nurses with an advanced tool to better monitor blood flow during free flap procedures, Cook Medical has launched the Doppler DP-M350 Blood Flow Monitor, a medical device at the forefront of…
March 18th, 2013
Bloomington, Ind.— Cook Medical has launched a suite of salivary duct access products that offer minimally invasive options for the treatment of obstructive salivary gland disease. Minimally invasive treatment of obstructive salivary gland disease can reduce the need for invasive…
January 28th, 2013
Bloomington, Ind. — The only FDA approved ureteral access sheath that has two options for placement is now available in the United States. The Flexor©Parallel™ Rapid Release™ Ureteral Access Sheath allows urologists to use a single wire guide that functions as…
January 2nd, 2013
Bloomington, Ind. — The healthcare industry is adopting a set of standards to make supply chain easier for hospitals and Cook Medical is ready for the change. Almost ten years ago, Cook Medical realised all of its manufacturing companies were using…
November 15th, 2012
Bloomington, Ind. — Cook Medical has received U.S. Food and Drug Administration (FDA) marketing approval for the first devices in its Zilver® PTX® Drug-Eluting Peripheral Stent portfolio, company officials reported today. It’s the first time the FDA has approved a drug-eluting…
October 12th, 2012
Las Vegas, Nevada — Three-year data from the Zilver® PTX® Randomized Controlled Trial of Paclitaxel-Eluting Stents for Femoropopliteal Disease from Cook Medical demonstrate 70.7 percent primary patency in the superficial femoral artery (SFA) at 36 months for patients treated with the…
September 28th, 2012
Baesweiler, North Rhine-Westphalia — Cook Medical, a world leader in minimally invasive medical technologies, today announced a major development in the extension of its European operations with the opening of a new €15m distribution center in Baesweiler, Germany. Located less than…
September 10th, 2012
Washington, D.C. — Cook Medical, a world leader in minimally invasive medical device technology, has launched its new Otolaryngology-Head and Neck Surgery (OHNS) clinical division to bring the benefits of the company’s devices for non-surgical procedures to a new group…
August 13th, 2012
Bloomington, Ind. — Previously unreleased three-year data from the Zilver PTX Randomized Controlled Trial of Paclitaxel-Eluting Stents for Femoropopliteal Disease indicate that Cook Medical's paclitaxel-eluting peripheral vascular stent demonstrated 83.0 per cent freedom from TLR at 36 months in the…
July 25th, 2012
Bloomington, Ind. — Reinforcing its commitment to advancing the field of interventional radiology (IR), Cook Medical today announced general availability of the Aprima™ Access Nonvascular Introducer Set, the first product in the Aprima drainage portfolio. It is a nonvascular access set…
June 25th, 2012
Bloomington, Ind. – Clinical investigators are for the first time examining the retrograde tibiopedal interventional approach, an endovascular technique that has the potential to reduce the rate of leg amputations by as much as 50 per cent1 in patients with critical limb…
May 21st, 2012
Bloomington, Ind. — Cook Medical has added a 25 gage needle to its EchoTip® ProCore™ line of fine needle biopsy (FNB) histology needles, which are designed for use in a procedure known as endoscopic ultrasound, or EUS. The EchoTip ProCore is…
April 9th, 2012
Bloomington, Ind. — As part of its ongoing commitment to patients needing emerging new cellular therapies, Cook Group has acquired the assets of General BioTechnology LLC and launched a new company, Cook General BioTechnology LLC (CGBT). Based in Indianapolis, CGBT…
February 7th, 2012
Bloomington, Ind. — Cook Medical's Cervical Ripening Balloon will now be available with a guiding stylet that offers added stiffness to facilitate placement when conditions for inducing labour are unfavourable. The addition of a stylet helps obstetricians position the balloon…
January 5th, 2012
Dublin – Minister for Jobs, Enterprise and Innovation Richard Bruton TD today announced that Cook Medical, the largest privately owned medical device company in the world, is to invest up to €16.5m over four years creating highly skilled positions in…
October 28th, 2011
Limerick, Ireland – Cook Medical's valuable place among the business community in Limerick has been recognised by Limerick Chamber at the inaugural Limerick Region Business Awards with Cook Ireland winning Company of the Year and Best Foreign Direct Investment Company…
September 9th, 2011
Munich, Germany – Cook Medical, a world leader in minimally invasive medical technologies, has launched the world’s first-ever stent designed and approved specifically to treat symptomatic iliofemoral venous outflow obstruction. The Zilver Vena Venous Self-Expanding Stent has received CE Mark…
July 14th, 2011
Bloomington, Ind. -- Continually striving to enhance patient care, Cook Medical recently announced significant updates to its Resonance Metallic Ureteral Stent System. First launched in 2007, the device now has an updated introducer system that includes a clear sheath for…
June 22nd, 2011
Warwickshire -- Cook Medical, a world leader in the development of advanced endovascular devices treating aortic disease, has sponsored a medical education programme created and administered by the British Society of Endovascular Therapy (BSET) to train British physicians in advanced…
June 7th, 2011
Bloomington, Ind. –Results from a multicentre European study1 suggest that a new, large-core biopsy needle designed for use with an endoscopic ultrasound (EUS) scope may help to overcome limitations of current EUS methods for biopsies of lesions in the gastrointestinal…
May 9th, 2011
Chicago, Ill. – An increasingly popular technique for removing lesions associated with Barrett’s oesophagus has been deemed a safe and effective treatment option in a study of more than 1,000 resections published this month in the European journal Endoscopy.¹ The…
May 4th, 2011
San Francisco, Calif. — A new version of the Liberator Locking Stylet with Beacon® Tip Technology, a distinct radiopaque tip marker, was unveiled at Heart Rhythm 2011, the 32nd Annual Scientific Sessions of the Heart Rhythm Society, and is now…
April 15th, 2011
Bloomington, Ind. – William Alfred Cook, founder of the Cook Group global network of companies and a pioneer in the development of life-saving minimally invasive medical device technology, died Friday at approximately 4:30 p.m. EDT at his Bloomington home of congestive…
March 24th, 2011
Bloomington, Ind. - Dan Sirota, who has served as global business leader for Cook Medical’s Interventional Radiology division since its inception in 2009, has been promoted to vice president of that business unit, the company said today. As vice president,…
February 16th, 2011
WINSTON-SALEM, N.C. — A new histology needle for endoscopic ultrasound (EUS) from Cook Medical is now on the market, giving physicians the ability to retrieve tissue samples from hard-to-reach regions within or adjacent to the GI tract with a minimally…
January 17th, 2011
Miami, Fla. – An investigational drug-eluting stent (DES) from Cook Medical showed sustained primary patency at two years compared to data collected at one year in the device’s prospective, randomised study, according to data presented today at the ISET 2010…