CIRSE comes to Denmark, home of WCE
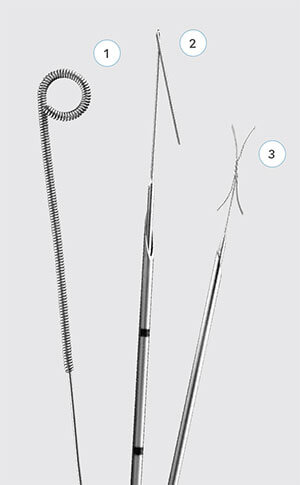
Cook Medical’s breast lesion localization devices:
1 MReye® Breast Lesion Localization Coil
2 Kopans Breast Lesion Localization Needle
3 X-Reidy Breast Lesion Localization Needle
In a report published in 2017, the World Cancer Research Fund International indicates that breast cancer is the most common cancer in women worldwide, with nearly 1.7 million new cases diagnosed in 2012 (second most common cancer overall). This represents about 12% of all new cancer cases and 25% of all cancers in women. It is the fifth most common cause of death from cancer in women.1 The top 10 countries with the highest incidence of breast cancer are (1) Belgium, (2) Denmark, (3) France, (4) the Netherlands, (5) the Bahamas, (6) Iceland, (7) the United Kingdom, (8) Barbados, (9) the United States, and (10) Ireland.1
Cook Medical offers a suite of breast lesion localization devices, which assist physicians in accurately locating breast lesions during surgery. The Kopans Breast Lesion Localization Needle, the MReye® Breast Localization Coil, and the X-Reidy Breast Lesion Localization Needle are intended for preoperative marking of nonpalpable breast lesions.
In addition to its standard needle, the Kopans Breast Lesion Localization Needle product line offers both echogenic and MR conditional options. Unlike standard, smooth-surfaced needles, EchoTip echogenic devices have textured surfaces. Textured surfaces help make the needles more visible under ultrasound.2,3 The EchoTip surface contains a band of microscopic dimpling around the full circumference of the needle shaft, close to the needle tip, for increased echo reflection. (Similarly, Cook’s textured etched tip needles contain finely roughened surfaces on the needle tip itself to help users detect the location of the needle tip for positioning.) The MReye versions of the Kopans needle are MR Conditional up to 1.5T. Kopans needles, in their smooth, echogenic and MR Conditional versions, are available in varying gages and lengths.
Additional features of the Kopans breast lesion localization needle include:
- A reference burnish mark, when visible just outside the needle hub on the spring-hook wire, provides visual assurance that the hook wire is within the needle tip during needle manipulation.
- The needle is marked in 1 cm increments for depth guidance during a procedure.
- The reinforced tip of the spring-hook wire serves as a visual and palpable landmark for the surgeon to gauge distance to the lesion during dissection and reduces the risk of severing the hook wire.
The MR conditional MReye® Breast Localization Coil acts as a marker to indicate the location of the lesion for the surgeon. It is created from a non-ferromagnetic material: a nickel-based super alloy called Inconel®,5 which has been shown to cause smaller, or fewer, image artifacts in MR imaging than stainless steel.4,6 The coil is MR Conditional up to 3T. The two-part positioning needle through which the coil is delivered contains EchoTip technology.
The X-Reidy Breast Lesion Localization Needle Set consists of a needle and a hook wire with a palpable 3 cm stiff segment just proximal to the X-shaped hook for easy palpation during surgery. The distinctive X-shaped hook provides fixation and is intended to limit migration. The remaining part of the hook wire is soft and flexible for patient comfort. A slide clamp is provided for use as a skin marker.
To learn more about the features of each of these breast lesion localization devices, view our product brochure here.
REFERENCES
1 Breast cancer statistics. World Cancer Research Fund International website. http://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/breast-cancer-statistics Accessed August 17, 2017.
2 Deam RK, Kluger R, Barrington MJ, et al. Investigation of a new echogenic needle for use with ultrasound peripheral nerve blocks. Anaesth Intensive Care. 2007;35(4):582-586.
3 Hocking G, Mitchell CH. Optimizing the safety and practice of ultrasound-guided regional anesthesia: the role of echogenic technology. Curr Opin Anaesthesiol. 2012;25(5):603-609.
4 MRI Tests Performed on the IDLC – Breast Lesion Localization Needle. 08/12/2009. Project conducted by Frank G. Shellock, Ph.D., Shellock R & D Services, Inc., 7511 McConnell Ave., Los Angeles, CA 90045. Data on file.
5 Inconel is a registered trademark of the Inco Alloys Corporation.
6 Stoeckel D. Nitinol – A material with unusual properties. Endovascular Update. 1998;(1):1-5.

Thousands of interventional radiologists from around the world gathered in Copenhagen from 16-20 September to hear about disease states, treatment modalities, and new technologies in the field.
CIRSE (Cardiovascular and Interventional Radiological Society of Europe) is an annual global congress designed to “improve patient care through the support of teaching, science, research, and clinical practice in the field of cardiovascular and interventional radiology.” Cook has been a part of CIRSE since its first congress in 1985.

The Cook Experience Centre featured a wall taking attendees from the history to the potential future of IR.
To augment the educational experience for attendees, Cook Medical provided three days of dynamic and interactive programs that focused on the latest treatment modalities in liver, venous, and arterial diseases. These informal presentations provide congress attendees an opportunity to gain access to the top minds in IR.
Presenters focused on therapies and techniques for embolisation, deep-vein thrombosis, below-the-knee disease, and more. Many shared some of their own cases that helped attendees gain knowledge on how to deal with similar situations they might face in their facilities.
The Cook Experience Centre offered an intimate setting for people to meet with leading specialists in the field, said Henrik, a product manager for BTK therapies. “Dr (Mariano) Palena spoke on the big stage on Sunday. It’s probably intimidating to ask questions when there are 300-400 people in the room,” he said. “But, the next day, he spoke in the Cook Experience Centre about tools and techniques for improving and optimising BTK success rates. The environment was comfortable and casual and attendees felt free to ask many questions.”
The physicians who presented in the Experience Centre were very gracious with their time, he added. “Dr Palena came 15 minutes early and stayed 20 minutes afterward to keep talking to the attendees. I could see it was very much appreciated.”
Watch CIRSE interview Dr Rick de Graaf on endovascular venous therapies for its Technology Spotlight in the Cook booth.

CIRSE 2017 took place in the Comwell Conference Center that adjoins Copenhagen’s Bella Sky hotel.
In addition to the presentations in the Experience Centre, Cook sponsored a well attended panel discussion in the main auditorium on drug elution, with some of the world’s leading specialists on peripheral arterial disease. Dr Michael Dake moderated sessions that focused on how to best serve patients by leaving the right thing behind in the superficial femoral artery.
“The panel spoke about vessel preparation, the differences between DCB and DES, and treating with drug-eluting technology in the SFA,” said Helle, product manager for PAD therapies. “They also discussed endovascular treatment versus bypass, cost effectiveness, and distinguishing the way studies present the evidence and how they may or may not compare to competitor studies.”
Cook also provided hands-on training sessions for medical students deciding on a specialty program. Participants learned about basic products and procedures and got a feel for the devices used to treat different areas of the body.
“The students learned about the steps and techniques involved with selected devices and were able to perform a procedure on a demo-model. They had the chance to ask as many questions as they would like,” said Wouter, an IR training coordinator leading one of the hands-on sessions. “They learned about IR, PAD, and venous devices and were able to get ideas and a good first impression of our products and procedures.”
Also in line with CIRSE’s call to “Impact the Future of IR,” the Cook Experience Centre featured a wall taking attendees from the history of IR (first recorded IV injection in 1665) to the father of IR (Charles Dotter’s video on angioplasty), to modern day therapies (first 3D printed liver tissue goes to market in 2014), and into an imagined future.
The Cook booth was a comfortable place for physicians to gather to have discussions over some of Copenhagen’s finest coffee. “We celebrated the return of CIRSE to Copenhagen with a modern booth design that allowed us to engage with attendees who visited our booth and Experience Centre,” said Casper, global brand marketing manager for IR. “We look forward to an equally successful congress at LINC 2018.”
We invite you to watch the video below to see the impact and power of collaboration in the past, present and into the future. Please join us on this journey.
5541629993001
brightcove
true
See you in Lisbon 22-26 September for CIRSE 2018!
 Four years ago, Tony had a serious case of liver disease, which caused ascites, an accumulation of fluid in the peritoneal cavity. He suffered painful abdominal swelling and had to go to the hospital once a week in order to have the fluid drained.
Four years ago, Tony had a serious case of liver disease, which caused ascites, an accumulation of fluid in the peritoneal cavity. He suffered painful abdominal swelling and had to go to the hospital once a week in order to have the fluid drained.
His doctor recommended that he undergo a TIPS procedure to help relieve his symptoms. Tony’s procedure was performed at IU Health in Indianapolis. A former quality engineer investigator at Cook Medical, Tony was all too familiar with the Rösch-Uchida Transjugular Liver Access Set that was going to be used during his procedure. The Rösch-Uchida Transjugular Liver Access Set is intended for transjugular liver access in diagnostic and interventional procedures.
“They gave me a local anesthetic, and I was awake and talking during most of the procedure,” said Tony. The TIPS procedure helped relieve the ascites, but during the procedure the doctor determined that Tony had such serious liver disease that he would need to get a liver transplant. He received his new liver in 2013.
“The TIPS procedure stabilized me and allowed me to survive long enough to get a new liver,” Tony said. “Without that liver transplant, I probably would not be here today.”
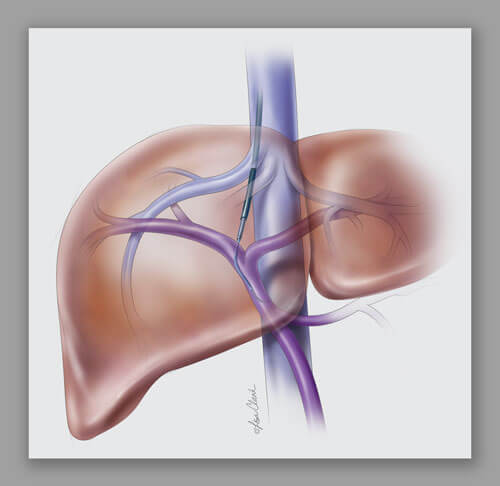 *What is a TIPS procedure?
*What is a TIPS procedure?
Transjugular intrahepatic portosystemic shunt (TIPS) is a procedure that creates new connections between two blood vessels in your liver.
The doctor inserts a catheter through your skin and into your jugular vein. Guided by x-ray imaging, the doctor guides the catheter into the hepatic vein in your liver. The doctor then creates a pathway through the liver for a stent that will connect the hepatic and portal systems. This new pathway helps to lower pressure in the portal system.
(Source: https://www.nlm.nih.gov/medlineplus/ency/article/007210.htm)

On 16 September, many of the brightest minds in interventional radiology will gather in Denmark, home of William Cook Europe, to participate in the 2017 Cardiovascular and Interventional Radiological Society of Europe (CIRSE) congress. CIRSE’s goal is to “improve patient care through the support of teaching, science, research, and clinical practice in the field of cardiovascular and interventional radiology.”
To help further that goal, Cook has organized a dynamic program at the Cook Experience Centre where attendees will have an opportunity to hear from some of the world’s leading interventional radiologists about the latest therapies and treatments for liver, venous, and arterial diseases. Click here for the full Experience Centre schedule.
On 18 September in Auditorium 1, Dr Michael Dake, USA, will chair a panel of experts to discuss the impact of drug elution in a symposium titled “Patient first: Leave the right thing behind.” He will be joined by a powerhouse of SFA experts, including Dr Fabrizio Fanelli, Sapienza University of Rome, Italy; Dr Renu Virmani, CVPath Institute, Gaithersburg, Maryland, USA; Dr Koen Deloose, AZ Sint-Blasius Hospital, Dendermonde, Belgium; and Dr John Kaufman, Dotter Interventional Institute, Portland, Oregon, USA.
CIRSE’s theme for 2017 is “Impact the Future of IR.” As Philosopher George Santayana once said, “To know your future, you must know the past.” Cook invites all CIRSE attendees to visit the Cook Experience Centre to see some of the incredible innovations that have occurred throughout the history (and pre-history) of interventional radiology.
We have also projected future technological advances in the evolving field of medicine and look forward to hearing attendees’ thoughts on how the medical device industry can best help the patients of tomorrow.
Join us in Cook’s newly designed booth for a casual place to share ideas about the future of medicine over a cup of some of Copenhagen’s finest coffee. And for more on important moments in history and potential future trends in this field of medicine, watch our video:
5541629993001
brightcove
true




 Four years ago, Tony had a serious case of liver disease, which caused ascites, an accumulation of fluid in the peritoneal cavity. He suffered painful abdominal swelling and had to go to the hospital once a week in order to have the fluid drained.
Four years ago, Tony had a serious case of liver disease, which caused ascites, an accumulation of fluid in the peritoneal cavity. He suffered painful abdominal swelling and had to go to the hospital once a week in order to have the fluid drained. *What is a TIPS procedure?
*What is a TIPS procedure?