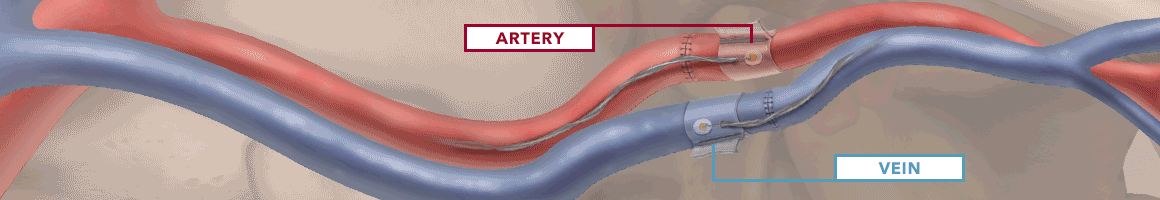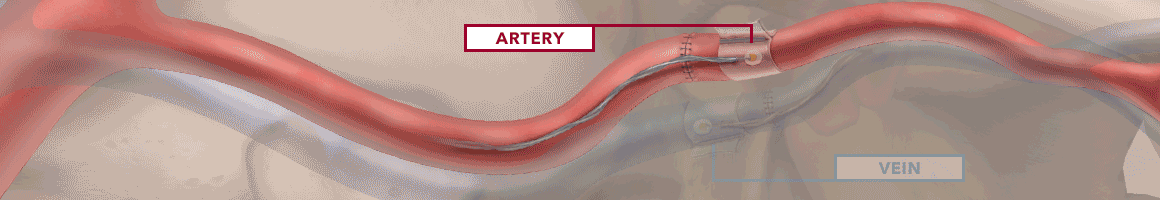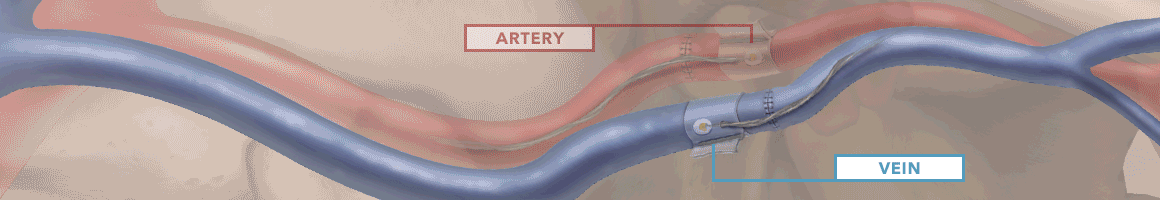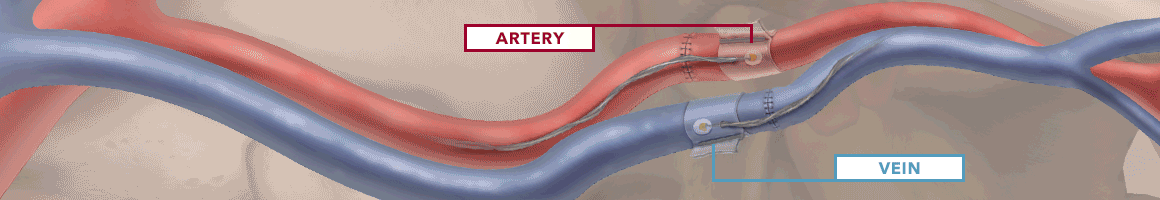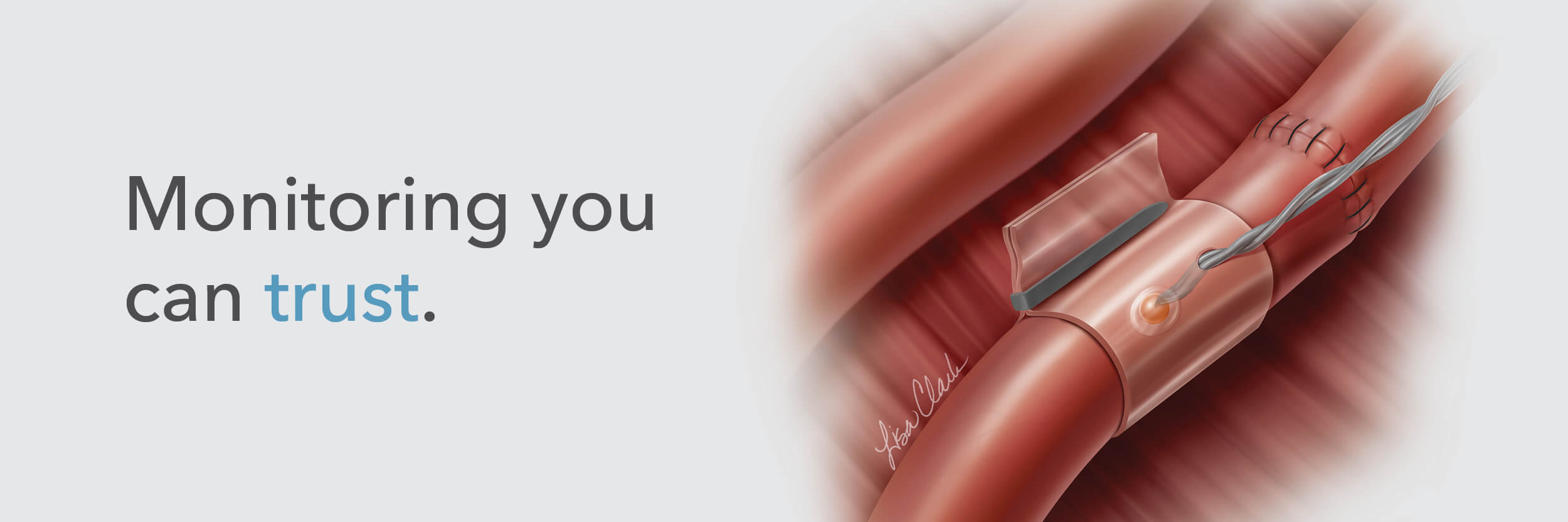
The Doppler monitor provides audible (primary) and visual (secondary) feedback of blood flow when connected to the implantable Cook-Swartz Doppler Probes and extension cables.
By allowing you to see and hear the presence or absence of blood flow, the Doppler system can alert you to flap failure in time to perform a salvage procedure.
Intended use
Monitoring blood flow in vessels intraoperatively and following reconstructive microvascular procedures, reimplantation, and free-flap transfers.
Request a demo
By clicking Submit, you agree to the terms and conditions for collecting and processing your personal information, as included in our customer data privacy notice.