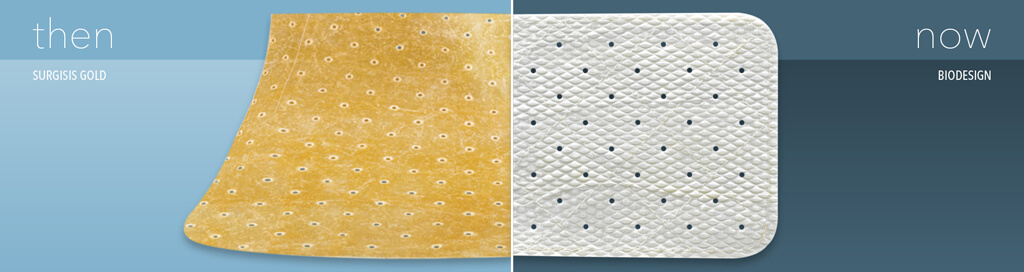
Biodesign® products from Cook Medical are constructed from small intestinal submucosa. But before our Biodesign products came into being, we had another line of small intestinal submucosa products. That product line was Surgisis®. In 2008, we created a new process for producing our small intestinal submucosa technology. Around that same time, we renamed the product line from Surgisis to Biodesign.
Our Biodesign product line for surgeons is similar to our Surgisis product line.
- Biodesign Hernia Graft
- Biodesign Hiatal Hernia Graft
- Biodesign Rectopexy Graft
- Biodesign Staple Line Reinforcement
- Biodesign Anal Fistula Plug Set
- Biodesign Fistula Plug Set
Why the changes?

Umesh, president of Cook
Biotech, listened to
surgeons’ suggestions.
When Cook Biotech first launched Surgisis products in 1998, they received positive feedback from surgeons. Umesh Patel, the president of Cook Biotech, remembers it clearly. Umesh was part of the team that first developed products from small intestinal submucosa. He and the Cook Biotech team worked hard to create Surgisis products, which were the first small intestinal submucosa products for surgeons.
‘We heard surgeons talking about how it could be improved’, says Umesh. ‘We’re always interested in improving our product.’ His team took a closer look at the product. They saw room for improvement in its handling characteristics and its biologic attributes. ‘We wanted to make a purer biomaterial. Lipids don’t have a place in the healing process. So, we reduced the amount of lipid in the material while maintaining other components that do have a place in the healing process.’
Then, they performed nonclinical studies. They saw more robust blood vessel ingrowth into the new material.1 They found that the new material rehydrated faster.1 In order to improve the operative performance, they included a quilting process.
‘We were satisfied once we saw the results of the clinical performance’, says Umesh. ‘We still continue to focus on improving our products and developing new ones.’
Learn more about small intestinal submucosa.
1 Data on file at Cook Biotech