
There are surprising moments in history that can have a profound impact on the advancement of medical device technology. Flexor technology was conceived from one of these seemingly ordinary circumstances.
In 1965, a casual conversation between physicians in the cafeteria of the UCLA Medical Center in California, USA, led to a substantial innovation in minimally invasive procedures. As Drs. Richard Hoffman and Donald Desilets sat together drinking sodas, they realized a straw-like pathway was the tool they needed to access a vessel without exchanging wires and catheters through tissue.
The first sets of catheter sheaths were homemade by Desilets and Hoffman from DuPont Mylar® soda straws. Shortly after, the physicians worked with Cook to produce the very first introducer sheath, which was fabricated from the same Mylar material as the straws. In 1969, thin-walled Teflon® replaced the Mylar.
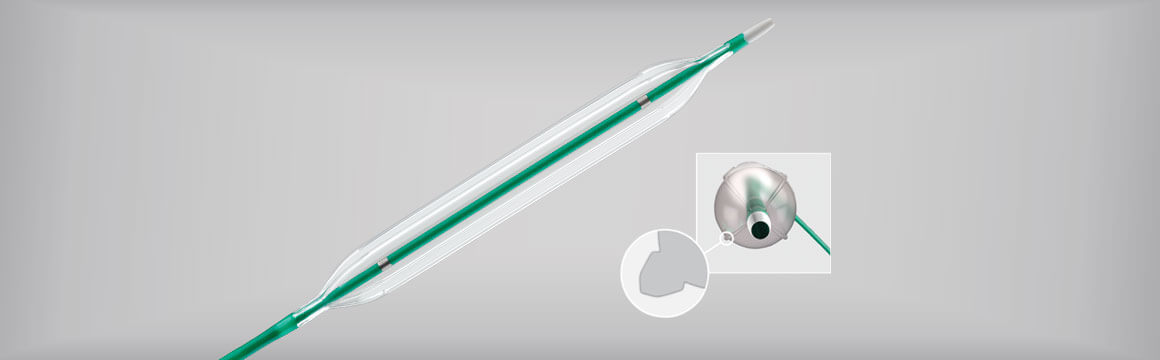
Decades later, as procedures evolved, clinicians desired kink-resistant, longer, soft-tipped sheaths to support the challenges of device delivery. Cook delivered on that need by developing the Flexor guiding sheath to help physicians access a vessel without exchanging wires and catheters through the tissue.
“Flexor guiding sheaths provide a reliable track from access point to treatment point, facilitate device exchanges, and provide support for the treatment products, like balloons and stents,” said Rikke Matthiesen, a product manager at William Cook Europe.
Flexor is an established technology that’s been on the market since 1991. Over time, this core technology product has evolved to offer nine Flexor “families” with a broad range of sizes, lengths, and designs adding up to more than 100 variations in EMEA alone. Flexor technology is currently used for aortic and peripheral applications, including for carotid, iliofemoral, radial, renal, and below-the-knee interventions.
Presenting a reliable tool for carotid intervention
Cook offers multiple options of Flexor guiding sheaths for iliofemoral procedures
Flexor provides options for renal interventions
Treating below the knee
Mylar is a registered trademark of DuPont Teijin Films.
Teflon is a registered trademark of E.I. du Pont de Nemours and Company.
Physicians facing tough lesions now have a balloon angioplasty catheter in their bag that applies focal force along four longitudinal elements.1
The Advance Enforcer 35 challenges lesions with focal force. Its construction uniquely incorporates four elements that transfer focal force to the lesion upon inflation.
The Advance Enforcer balloon was designed to provide concentration of force within a specific area. Along the elements of this balloon, a greater amount of outward force is applied in contrast to a uniform circumferential distribution of force with other angioplasty balloons.
Benchtop testing demonstrated that along the Enforcer balloon elements, up to 465% more force was applied compared to any specific area of a standard PTA balloon.1
Dr Andrew Holden, Auckland City Hospital in New Zealand, conducted a first-in-man study specific to treatment of hemodialysis access patients. In April 2016, Dr Holden presented six-month results of his study during the Charing Cross International Symposium.
Data from the study indicate that the Advance Enforcer 35 has good acute performance and can successfully treat challenging lesions, he said.2
According to Dr Holden, there was an unmet need to create a new scoring balloon. “Stenoses in haemodialysis access circuits are often resistant to angioplasty using standard .035 inch guidewire-compatible devices,” he said.
In addition, ancillary procedures such as scoring/cutting balloons or high-pressure balloons are often required. These ancillary procedures incur additional cost and procedural time, upsizing of access sheath, and a change of wire guide.
The Advance Enforcer 35 comes in lengths of 50, 80, and 135 cm, and diameters of 6, 8, 10, and 12 mm. It is approved for sale in the United States and Europe.
Dr Holden is a paid consultant of Cook Medical.
1. Test Report Cook® Incorporated. Element Effectiveness. Project Number: 008-01-10-A.
2. Holden A. Advance® Enforcer™ 35 focal-force PTA balloon catheter: 6-month results. Presented at: Charing Cross International Symposium; April 26-29, 2016; London UK.

Dr. Christopher Boyes
The following story is an example of a coordinated approach to limb salvage instituted at Sanger Heart and Vascular Institute in Charlotte, North Carolina.
One of the most devastating consequences of advanced peripheral arterial disease (PAD) is critical limb ischemia (CLI), a severe blockage of the arteries that reduces blood flow to the extremities. CLI results in severe pain, skin ulcers, and, if left untreated, gangrene.
Unfortunately, the first line of treatment is often below-the-knee (BTK) or above-the-knee (ATK) amputation. While amputation may sometimes be the best option for the patient, it is important that the physician conduct a vascular workup beforehand to ensure an endovascular procedure isn’t a viable alternative.
Tracking total amputation rates due to diabetes and/or PAD in the general population has proven to be difficult. Reports have shown a 23% increase in amputation rates in the diabetic population since 19881 with a total annual cost of approximately $8.3 billion, not including prosthetic or rehabilitation costs2. Studies have shown that the total lifetime healthcare costs for an amputee is more than $500,000 per person, which is double the lifetime medical cost for the average person3,4. When you consider the high 5-year mortality associated with amputations, this number is even more staggering.
In addition to the high costs, there is a clear trend for recurrence of amputations, and the mortality rates within one year of CLI diagnosis and amputation in the Medicare population is 40.42%5.
Many interventionalists “consider endovascular treatment of CLI ‘voodoo medicine,’” explained Dr. Christopher Boyes, vascular surgeon at Sanger Heart & Vascular Institute in Charlotte, North Carolina. “When did it become accepted that an amputation is the ‘safe’ option for patients with CLI?”
According to Dr. Boyes, there are several factors that have led to this drastic standard of care. But, he said, with education, training, and a new CLI treatment algorithm, endovascular interventions could become the accepted first line of therapy.
But, it’s not all about patency rates. “It’s important to note that focusing solely on the intervention and not the external variables that account for limb salvage is a mistake,” he said. “We must take a multidisciplinary approach to limb salvage.”
The Sanger limb salvage program was started two years ago and focuses on points of entry, which include podiatrists, wound-care physicians, vascular surgeons, orthopedists, and emergency room staff who are often the first to see patients with CLI symptoms. Testing for these patients should include measuring toe brachial index (the ratio between toe pressure and the highest of the two brachial pressures) and HbA1c levels (used to diagnose diabetes and prediabetes).
If patients present with nonhealing wounds, they should be referred to a vascular specialist as soon as possible. “The earlier the treatment, the better the chances are for limb salvage,” Dr. Boyes said.
Following an endovascular procedure, patients should be educated about follow-up care, including the importance of wearing proper socks and shoes, orthotic care, and risk-factor modification, such as antiplatelets, statins, smoking cessation, diabetic control, and checking feet for reoccurrence of the wound.
To ensure the process is sufficiently managed, the limb salvage program needs to be viewed as a team effort with a navigator coordinating the process to ensure the team stays on the same page.
“Communication is key to improving the process, from beginning to end,” Dr. Boyes said. “Through better standards and reporting processes, we hope that a collaborative approach to the management of CLI and amputation prevention will become the new standard of care within the next five years.”
Dr. Christopher Boyes is a paid consultant of Cook Medical.
1. Centers for Disease Control and Prevention NCfHS. “Number (in Thousands) of Hospital Discharges for Nontraumatic Lower Extremity Amputation with Diabetes as a Listed Diagnosis, United States, 1988-2009.” Atlanta, GA: Centers for Disease Control and Prevention National Center for Health Statistics; 2012 [cited 2012 April 2,]; Available from: cdc.gov/diabetes/statistics/lea/fig1.htm.
2. HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2009.
3. Blough DK, Hubbard S, McFarland LV, Reiber GE, Smith DG, Gambel JM. “Prosthetic Cost Projections for Servicemembers with Major Limb Loss From Vietnam and OIF/OEF.” Journal of Rehabilitation Research and Development; 2010;47(4):387‐402.
4. Alemayehu B, Warner KE. “The Lifetime Distribution of Health Care Costs.” Health Services Research; 2004;39(3):627‐42.
5. Baser O, Verpillat P, Gabriel S, et al. Prevalence, incidence, and outcomes of critical limb ischemia in the US Medicare population. Vascular Disease Management Web site. http://www.vasculardiseasemanagement.com/content/prevalence-incidence-and-outcomes-critical-limb-ischemia-us-medicare-population. Published Feb. 8, 2013. Accessed March 16, 2017.
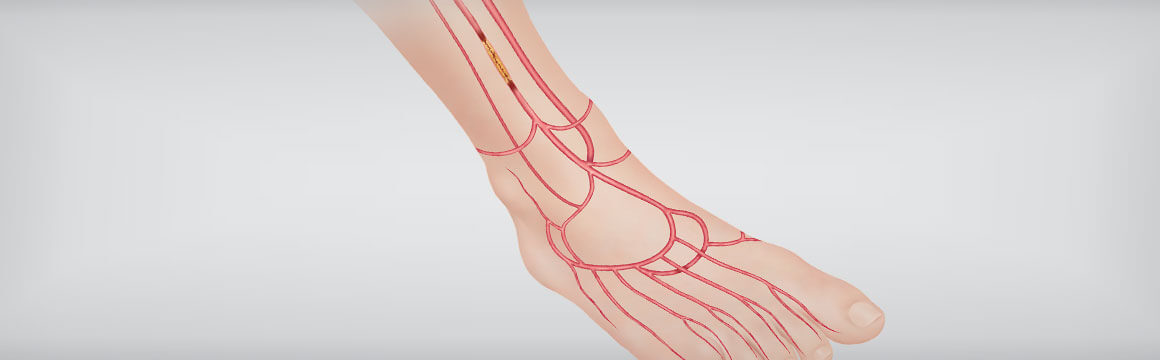
Patients suffering from critical limb ischemia (CLI) are often a difficult population to treat due to the likelihood of significant comorbidities including lesions that may be extremely difficult to cross. Increasingly, physicians are crossing these difficult lesions from a tibiopedal retrograde approach.
In order to determine the technical access and crossing success rate and safety of these procedures, Cook Medical sponsored the world’s first prospective, multicenter tibiopedal study.
In December, the Journal of Endovascular Therapy (JEVT) published results of the study titled “Tibiopedal Access for Crossing of Infrainguinal Artery Occlusions: A Prospective Multicenter Observational Study.”
According to Mark Breedlove, vice president and global business unit leader of Peripheral Intervention, “We wanted to learn more about new crossing procedures and techniques and find out what works and what does not,” he explained. “The study was designed to provide physicians with safety and technical success measures to potentially help expand their ability to safely treat these difficult lesions and patient population.”
Results of the study indicate that technical tibiopedal access success was achieved in 93.4% of 197 patients and technical occlusion crossing success in 85.3% of the 184 successful tibial accesses. The access success rate was 92.4% after a failed antegrade access vs. 95.4% in those with a primary tibiopedal attempt. Crossing success was achieved in 82.8% after a failed antegrade access vs. 90.3% for patients with no prior antegrade attempt.
The majority of the study participants (67.0%) had previously experienced a failed antegrade attempt to cross the occlusion. Of these patients, 77.3% had combined access and crossing success from a tibiopedal access.
Minor complications related to the access site occurred in 5.6% of cases. No patient had access vessel thrombosis, compartment syndrome, or surgical revascularization.
The study’s authors determined that “tibiopedal access appears to be safe and can be used effectively for the crossing of infrainguinal lesions in patients with severe lower limb ischemia.”
Results of the study show that high retrograde access and crossing success may have prevented amputations for those who had previously undergone a failed antegrade procedure.
Here is principal investigator Dr. Craig Walker talking about the study:
5270396090001
brightcove
true



