Physicians facing tough lesions now have a balloon angioplasty catheter in their bag that applies focal force along four longitudinal elements.1
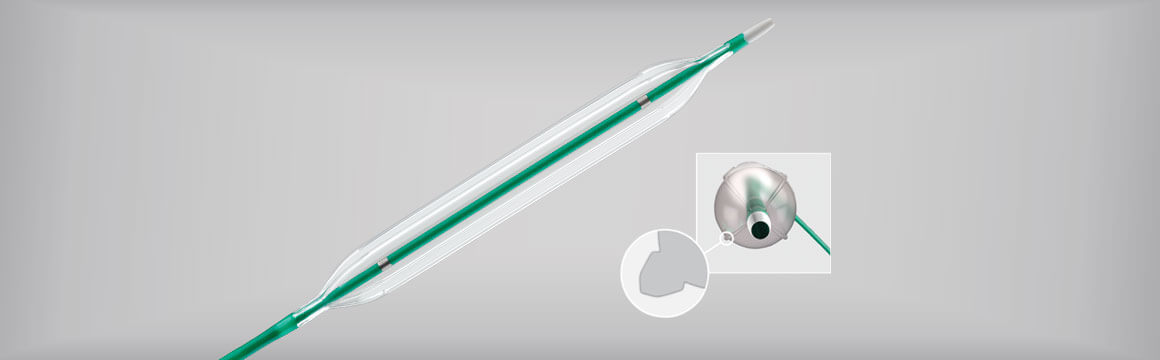
The Advance Enforcer 35 challenges lesions with focal force. Its construction uniquely incorporates four elements that transfer focal force to the lesion upon inflation.
The Advance Enforcer balloon was designed to provide concentration of force within a specific area. Along the elements of this balloon, a greater amount of outward force is applied in contrast to a uniform circumferential distribution of force with other angioplasty balloons.
Benchtop testing demonstrated that along the Enforcer balloon elements, up to 465% more force was applied compared to any specific area of a standard PTA balloon.1
Dr Andrew Holden, Auckland City Hospital in New Zealand, conducted a first-in-man study specific to treatment of hemodialysis access patients. In April 2016, Dr Holden presented six-month results of his study during the Charing Cross International Symposium.
Data from the study indicate that the Advance Enforcer 35 has good acute performance and can successfully treat challenging lesions, he said.2
According to Dr Holden, there was an unmet need to create a new scoring balloon. “Stenoses in haemodialysis access circuits are often resistant to angioplasty using standard .035 inch guidewire-compatible devices,” he said.
In addition, ancillary procedures such as scoring/cutting balloons or high-pressure balloons are often required. These ancillary procedures incur additional cost and procedural time, upsizing of access sheath, and a change of wire guide.
The Advance Enforcer 35 comes in lengths of 50, 80, and 135 cm, and diameters of 6, 8, 10, and 12 mm. It is approved for sale in the United States and Europe.
Dr Holden is a paid consultant of Cook Medical.
1. Test Report Cook® Incorporated. Element Effectiveness. Project Number: 008-01-10-A.
2. Holden A. Advance® Enforcer™ 35 focal-force PTA balloon catheter: 6-month results. Presented at: Charing Cross International Symposium; April 26-29, 2016; London UK.