 Physicians frequently highlight the lack of multicenter, prospective, randomized controlled trials that provide clear answers to clinical questions. These studies are difficult to do and require substantial commitment of resources and time to be successful. But when done well, these studies offer an unbiased result that offers meaningful information to the practicing clinician.
Physicians frequently highlight the lack of multicenter, prospective, randomized controlled trials that provide clear answers to clinical questions. These studies are difficult to do and require substantial commitment of resources and time to be successful. But when done well, these studies offer an unbiased result that offers meaningful information to the practicing clinician.
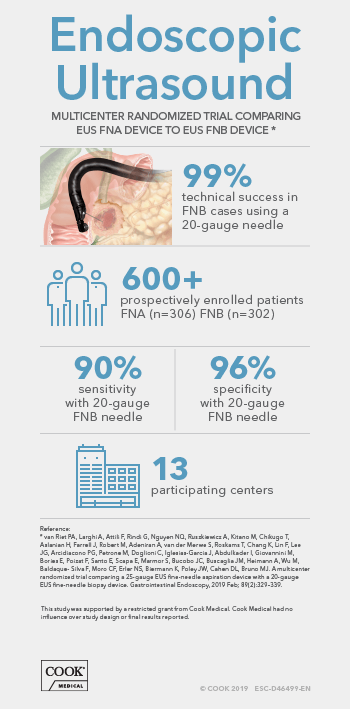
Endoscopic ultrasound (EUS) guided fine needle biopsy (FNB) has become the primary method for evaluating suspicious lesions in the pancreas and surrounding tissue, as well as staging disease progression in lung and digestive system cancers. Newer treatments and genetic characterization of cancers require more tissue than is necessary for a simple positive or negative diagnosis obtained from Fine Needle Aspiration (FNA) samples, but robust study data for FNB is lacking. Many of the published studies are retrospective reports, which potentially carry substantial risk of case selection bias. The prospective studies are often single-center cohort studies that limit generalizability of the results, or show improvement in cytological yield, but not histological yield. In fact, the 2017 ESGE Guideline does not favor either FNA or FNB due to a lack of well-designed studies.
Fortunately, that is changing. There is a new prospective, randomized controlled study conducted in 13 EUS centers worldwide in 9 countries (Australia, Belgium, France, Italy, Israel, Japan, Netherlands, Spain, and the United States). This study compared the widely used 25-gauge FNA needle to the 20-gauge ProCore FNB needle. It is one of the largest prospective randomized studies on FNA vs FNB sampling techniques ever conducted, enrolling 608 patients from February 2015 to September 2016.
While techniques were not standardized across all study centers, the diversity of practice and sample-handling show the results are not limited to a single method or skill set, or dependent on a dedicated expert pathologist.
The study prospectively enrolled consecutive patients undergoing EUS-guided tissue acquisition for a solid pancreatic lesion, lymph node, or other solid or submucosal lesion. Patients were randomized into two groups. One group had samples obtained with the 25-gauge FNA needle, the other with the 20-gauge ProCore FNB needle. Samples were collected in 3 separate vials to evaluate the effective number of passes to obtain a diagnosis, and samples were handled according to local practice, which varied between centers and countries. Pathologists recorded if a diagnosis was obtained from cytology, histology, or with a combination of techniques.
The overall study results showed both needles were very safe, with only 5 minor adverse events equally spread between both groups (3 FNA, 2 FNB). Over 3 passes were more frequently undertaken in the FNA group. Rapid on-site pathological assessment (ROSE) was performed in only 17% of all procedures, and more often in the 25-gauge FNA group. Also, technical success (defined as ability to obtain tissue from the target lesion) was 99% using the 20-gauge FNB needle even though a significant number of lesions were sampled from an angulated endoscope position, including pancreatic head masses.*
The authors concluded that the 20-gauge FNB needle (ProCore design) consistently outperformed one of the most widely used 25-gauge FNA needles in terms of histological yield and diagnostic accuracy, in pancreatic as well as non-pancreatic lesions and independent of the number of passes performed. The consistency of its diagnostic benefit among the 13 participating centers supports the general applicability of these findings.*
What does this study mean to the clinician who wants to provide the best care options for their patient? First, overall performance of the 20-gauge ProCore FNB needle is consistent across a variety of sites and operators, despite different specimen-processing methods and pathology-assessment techniques. It allows clinicians to access almost any position, even those anatomical locations that typically require difficult scope positions. Sufficient tissue can be obtained in almost all cases, usually in fewer than 3 passes, and ROSE is not necessary to assure adequacy of the sample. Overall, the 20-gauge ProCore offers the clinician better overall performance, sufficient tissue to assure an accurate diagnosis, and offers the benefits of a large-bore needle without the loss of flexibility, access, or risk of adverse events typically associated with larger needles.
In an era where evidence-based medicine increasingly demands high quality, prospective, randomized controlled trials, this ASPRO study meets that level-of-evidence requirement. And, although some may argue that the study did not directly compare the 20-gauge ProCore with other “core” biopsy needles, this new data establishes a benchmark of objective performance for the 20-gauge ProCore needle against which other FNB needles should be measured.
* van Riet PA, Larghi A, Attili F, Rindi G, Nguyen NQ, Ruszkiewicz A, Kitano M, Chikugo T, Aslanian H, Farrell J, Robert M, Adeniran A, van der Merwe S, Roskams T, Chang K, Lin F, Lee JG, Arcidiacono PG, Petrone M, Doglioni C, Iglesias-Garcia J, Abdulkader I, Giovannini M, Bories E, Poizat F, Santo E, Scapa E, Marmor S, Bucobo JC, Buscaglia JM, Heimann A, Wu M, Baldaque- Silva F, Moro CF, Erler NS, Biermann K, Poley JW, Cahen DL, Bruno MJ. A multicenter randomized trial comparing a 25-gauge EUS fine-needle aspiration device with a 20-gauge EUS fine-needle biopsy device. Gastrointestinal Endoscopy. 2019 Feb; 89(2):329-339
This study was supported by a restricted grant from Cook Medical. Cook Medical had no influence over study design or final results reported.