 Understanding the effects on aortic remodeling and malperfusion.
Understanding the effects on aortic remodeling and malperfusion.
This blog content derives from the January 2016 EVT Europe Supplement: Global Approaches To Type B Dissection article written by Jonathan Sobocinski, MD, PhD; Nuno V. Dias, MD, PhD; Rachel Clough, MD, PhD; and Stéphan Haulon, MD, PhD
Malperfusion of aortic branches and aortic rupture are the two most feared complications in the acute phase of a type B aortic dissection. When such complications occur, stent graft deployment should be considered with the proximal landing zone in a healthy, nondissected aorta.1-3 The coverage of the main proximal intimal tear redirects the aortic flow toward the true lumen and thus promotes a drop of pressure within the false lumen. Thoracic stent grafting (TEVAR) has been associated with encouraging early outcomes for the treatment of acute complicated type B aortic dissection,4,5 but questions still remain regarding the mid- and long-term results.6 Initial successful treatment with TEVAR is not necessarily associated with favorable remodeling of the dissected aorta during follow-up.7 Only few exhaustive anatomical analyses of the aorta following TEVAR for acute type B aortic dissection have been performed so far.7-11 TEVAR generally induces positive aortic remodeling, but this is usually limited to the aortic segment covered by the stent graft; frequently, the outcomes of the distal thoracic and abdominal aorta remain of concern.12
The additional implantation of a self-expanding bare stent in the aortic true lumen, distally to the proximal stent graft(s), was proposed in 2005. This composite device design approach, also known as STABLE (staged aortic and branch vessel endoluminal repair), aimed to enhance global aortic remodeling, especially in the area of the abdominal aorta, and to improve the management of visceral/renal/lower limb malperfusion in the acute phase.13 First, the proximal stent graft is positioned, then intraprocedural angiography is performed to assess if there is insufficient expansion of the true lumen, continuous retrograde perfusion of the false lumen, or evidence of malperfusion of arterial branches originating from the true lumen. If any of these features is present, then a distal bare stent may be used (Figure 1).14
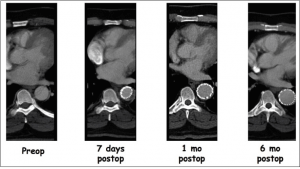
Figure 1. A patient with acute complicated type B aortic dissection was treated with a composite device design (stent graft and bare stent). Successive CTAs showing remodeling of the thoracic aorta, including expansion of the true lumen and thrombosis of the false lumen.
Variations of the endovascular techniques described in the STABLE trial have been reported. He et al have suggested that if the proximal stent graft is deployed first, then the distal end of the stent graft is landed in the diseased aorta, which has the risk of causing further aortic dissection or other structural damage. They therefore advocate that the distal bare stent is placed first, at the intended distal landing zone, followed by the proximal stent graft.15 Hofferberth et al reported the use of balloon-driven expansion of the true lumen following placement of the bare-metal stent below the stent graft to remove any residual flow in the false lumen and achieve complete true lumen expansion. This technique is called the stent-assisted balloon-induced intimal disruption and relamination in aortic dissection repair (STABILISE).16
AORTIC REMODELING
Long-term results from the IRAD registry indicate that at 5 years, more than 60% of patients develop aortic growth or formation of a new aneurysm after endovascular repair of acute complicated type B aortic dissection.17 The implantation of a bare stent distal to the stent graft, to support true lumen expansion within the thoracoabdominal aorta, can be performed to promote remodeling of the dissected aorta. Improved aortic remodeling has been associated with a reduced risk of late aortic complications during follow-up. In a prospective, nonrandomized, multicenter feasibility study (STABLE 1 study), 86 patients with acute and subacute type B aortic dissections (within 90 days of symptom onset) underwent endovascular repair with a composite device design (Zenith TX2 Endovascular Graft and Zenith Dissection Endovascular Stent, Cook Medical). The procedure appeared to be safe, and good clinical outcomes were reported. Two years after the initial procedure, positive remodeling was observed in both the thoracic and the abdominal aorta.18,19
Patterson et al gathered 16 studies that reported the results of aortic remodeling after TEVAR with stent graft alone in aortic dissection.20 In their study, patients with acute (≤ 14 days) and chronic (> 14 days) aortic dissections were analyzed separately. Patients with infrarenal extension of the aortic dissection experienced less aortic remodeling. This may be because TEVAR did not cover distal, secondary re-entry tear(s). The main limitation of the study was that no standard analysis was performed to evaluate aortic remodeling. The lack of clear reporting standards means that the clinical evidence is heterogeneous, making it difficult to evaluate the impact of TEVAR on aortic remodeling following aortic dissection. Also, the additional role of the bare stent in aortic remodeling has not yet been fully determined, and questions surrounding the use of the bare stent focus largely on its application and utility, compared to TEVAR alone.
We performed a retrospective analysis including 84 patients that compared aortic morphological and clinical outcomes of patients undergoing endovascular repair with stent graft(s) alone (TEVAR, 45 patients) and patients who were treated in the STABLE 1 study with the composite device design (STABLE, 39 patients).21 The analysis focused on aortic remodeling at 1 year. Only patients with complicated acute dissection (≤ 14 days) and with available CT scans at preprocedure and 1-year follow-up were included. The study provided a thorough aortic morphological analysis including lengths, diameters, volumes, intimal tears, and lumen patency, performed on a dedicated three-dimensional workstation. Remodeling of the dissected aorta was assessed by changes in diameter and volume of the false lumen, true lumen, and total lumen and also patency of the false lumen on the preoperative and latest CT angiography performed during follow-up.
Both groups presented with largely comparable preoperative medical conditions and similar extent of aortic dissection; however, the false lumen volume was significantly larger in the STABLE group in both the thoracic and abdominal aorta. The length of aorta covered by stent grafts was not statistically different in both groups (167 ± 47 mm in STABLE patients; 184 ± 49 mm in patients with stent graft alone; P = .11).
Both groups exhibited extensive thrombosis of the thoracic false lumen after endovascular repair, although complete false lumen thrombosis occurred less frequently in the abdominal aorta. Although there was no statistical difference between the two groups in the proportion of patients who experienced > 10% in changes to the thoracic or abdominal total lumen diameter or volume following endovascular repair through 12 months, there was a trend toward larger expansion of the true lumen, and more importantly, shrinkage of the false lumen in the abdominal aorta in patients treated with the composite device design. As a reminder, only patients who survived at 12 months were included in this study. Rupture, conversion, and reintervention rates were comparable between the two groups.
Previous publications have already confirmed that the deployment of a bare-metal stent within the distal aorta is safe and does not expose patients to a higher risk of complications.2,17,22 Of note, in the setting of aneurysmal dilatation of the dissected aorta in the presence of a bare stent within the true lumen, fenestrated stent grafting can be performed.23
Aortic growth is frequently observed in patients who receive endovascular management in the acute phase.17,19,22 More extensive coverage of the thoracic aorta usually results in early thoracic aorta remodeling and greater false lumen thrombosis, at least at the level of the stent graft.24,25 The additional risks of extensive coverage (such as spinal cord ischemia) must be balanced against the risk of long-term aortic growth in each individual patient.
MALPERFUSION
Malperfusion in patients with type B aortic dissection is associated with 30-day mortality and morbidity rates of 2.7% and 51.8%, respectively.26 Most cases of aortic branch malperfusion in aortic dissection involve a dynamic mechanism (true lumen collapse above or at the level of the origin of the branch, with change in the flap position during the cardiac cycle); few cases of aortic branch malperfusion result from a static mechanism only.27 In some cases, it can be difficult to make an accurate diagnosis of malperfusion due to incongruity between the clinical and imaging signs; also, there are no clear guidelines on when an intervention is warranted (Figure 2).
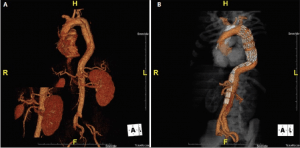
Figure 2. A patient presented with acute type B aortic dissection complicated with visceral and lower limb malperfusion (preoperative CTA)(A). The postoperative CTA showed that the use of a composite device design promoted sufficient true lumen expansion to correct the malperfusion syndrome without additional selective stenting or fenestration (B). At this early stage, the distal false lumen was still patent.
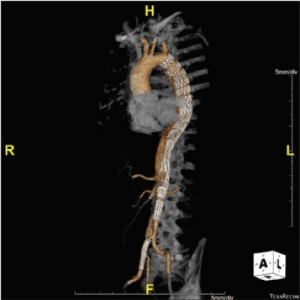
Figure 3. In the latter case, despite good reexpansion of the true lumen after the deployment of a composite device design, residual static malperfusion of the right common iliac artery required additional stenting.
The correction of competitive flow and pressure between the true and the false lumens is usually obtained after coverage of the main proximal entry tear (frequently located around the aortic isthmus) with a stent graft. We only consider selective visceral/renal/lower limb artery stenting or flap fenestration after exclusion of the primary tear in the proximal descending thoracic aorta. After deployment of the stent graft, an angiogram is performed to evaluate the flow within the true lumen and whether any malperfusion remains. It will also depict large secondary tears that can generate dynamic malperfusion. In our practice, the coverage of the descending thoracic aorta is extensive only when malperfusion persists after deployment of the proximal stent graft and/or when aortic rupture is suspected. It always needs to be balanced against the risk of spinal cord ischemia. When a secondary entry tear is located in the abdominal aorta or close to the visceral/renal arteries, flap fenestration can be proposed to relieve malperfusion. In addition, the deployment of a bare stent can improve true lumen expansion and the quality of distal flow and thus reduce the risk of residual malperfusion after TEVAR and the need for additional revascularization (Figure 3).
In a previous series of 52 patients with acute type B aortic dissection treated in Lille, Caen, and Malmö between 2004 and 2011, 17 out of 22 patients presenting with malperfusion required additional revascularization after stent graft deployment.22 In the STABLE 1 study of the composite device design, 9 of 40 patients (23%) required adjunctive branch vessel stenting during the index procedure.18 From the morphological study of TEVAR and STABLE as previously described, we learned that the use of an additional aortic bare stent seems to promote aortic remodeling by increasing true lumen expansion and false lumen shrinkage in the abdominal aorta. Further analysis is required to determine the impact of the composite device approach on outcomes related to malperfusion. The origin of the visceral/renal/lower limb arteries should be noted before stent placement, because previous studies have shown there is reduced flow in vessels originating from the false lumen after endovascular repair.28
Taken together, placement of a bare-metal stent below a proximal stent graft in patients with type B aortic dissection appears to provide favorable short- and midterm outcomes, and treatment should be tailored to each individual patient, although longer-term outcomes are required to better understand the effect of this approach on aortic remodeling and malperfusion.
Jonathan Sobocinski, MD, PhD, is with the Aortic Centre, Vascular Surgery, Hôpital Cardiologique, Lille University Hospital, Lille, France.
Nuno V. Dias, MD, PhD, is with the Vascular Center Malmö-Lund, Skåne University Hospital, Malmö, Sweden.
Rachel Clough, MD, PhD, is with the Aortic Centre, Vascular Surgery, Hôpital Cardiologique, Lille University Hospital, Lille, France.
Stéphan Haulon, MD, PhD, is with the Aortic Centre, Vascular Surgery, Hôpital Marie Lannelongue, Le Plessis-Robinson, France.
Get stories like these sent to your email.
Sign up for our quarterly email newsletter to receive physician stories, product news, training opportunities and more in your inbox.
Drs. Sobocinski, Clough, Dias and Haulon are paid consultants of Cook Medical.
-
Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol. 2013;61:1661-1678.
-
White RA, Miller DC, Criado FJ, et al. Report on the results of thoracic endovascular aortic repair for acute, complicated, type B aortic dissection at 30 days and 1 year from a multidisciplinary subcommittee of the Society for Vascular Surgery Outcomes Committee. J Vasc Surg. 2011;53:1082-1090.
-
Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation. 2010;121:e266-e369.
-
Zeeshan A, Woo EY, Bavaria JE, et al. Thoracic endovascular aortic repair for acute complicated type B aortic dissection: superiority relative to conventional open surgical and medical therapy. J Thorac Cardiovasc Surg. 2010;140:S109-S115.
-
Rogers AM, Hermann LK, Booher AM, et al. Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation: results from the international registry of acute aortic dissection. Circulation. 2011;123:2213-2218.
-
Steuer J, Eriksson MO, Nyman R, et al. Early and long-term outcome after thoracic endovascular aortic repair (TEVAR) for acute complicated type B aortic dissection. Eur J Vasc Endovasc Surg. 2011;41:318-323.
-
Investigators VR. Mid-term outcomes and aortic remodeling after thoracic endovascular repair for acute, subacute, and chronic aortic dissection: the VIRTUE Registry. Eur J Vasc Endovasc Surg. 2014;48:363-371.
-
Melissano G, Bertoglio L, Rinaldi E, et al. Volume changes in aortic true and false lumen after the “PETTICOAT” procedure for type B aortic dissection. J Vasc Surg. 2012;55:641-651.
-
Resch TA, Delle M, Falkenberg M, et al. Remodeling of the thoracic aorta after stent grafting of type B dissection: a Swedish multicenter study. J Cardiovasc Surg (Torino). 2006;47:503-508.
-
Schoder M, Czerny M, Cejna M, et al. Endovascular repair of acute type B aortic dissection: long-term follow-up of true and false lumen diameter changes. Ann Thorac Surg. 2007;83:1059-1066.
-
Yang CP, Hsu CP, Chen WY, et al. Aortic remodeling after endovascular repair with stainless steel-based stent graft in acute and chronic type B aortic dissection. J Vasc Surg. 2012;55:1600-1610.
-
Eggebrecht H, Nienaber CA, Neuhauser M, et al. Endovascular stent-graft placement in aortic dissection: a meta-analysis. Eur Heart J. 2006;27:489-498.
-
Mossop PJ, McLachlan CS, Amukotuwa SA, Nixon IK. Staged endovascular treatment for complicated type B aortic dissection. Nat Clin Pract Cardiovasc Med. 2005;2:316-321.
-
Kische S, D’Ancona G, Ortak J, et al. Complicated type B aortic dissection should not be treated with uncovered stents: a lesson not yet learned. Ann Vasc Surg. 2015;29:e813-e847.
-
He H, Yao K, Nie WP, et al. modified petticoat technique with pre-placement of a distal bare stent improves early aortic remodeling after complicated acute stanford type B aortic dissection. Eur J Vasc Endovasc Surg. 2015;50:450-459.
-
Hofferberth SC, Nixon IK, Boston RC, et al. Stent-assisted balloon-induced intimal disruption and relamination in aortic dissection repair: the STABILISE concept. J Thorac Cardiovasc Surg. 2014;147:1240-1245.
-
Fattori R, Montgomery D, Lovato L, et al. Survival after endovascular therapy in patients with type B aortic dissection: a report from the International Registry of Acute Aortic Dissection (IRAD). JACC Cardiovasc Interv. 2013;6:876-882.
-
Lombardi JV, Cambria RP, Nienaber CA, et al. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg. 2012;55:629-640.
-
Lombardi JV, Cambria RP, Nienaber CA, et al. Aortic remodeling after endovascular treatment of complicated type B aortic dissection with the use of a composite device design. J Vasc Surg. 2014;59:1544-1554.
-
Patterson BO, Cobb RJ, Karthikesalingam A, et al. A systematic review of aortic remodeling after endovascular repair of type B aortic dissection: methods and outcomes. Ann Thorac Surg. 2014;97:588-595.
-
Sobocinski J, Lombardi JV, Dias NV, et al. IF8: Volume analysis of true and false lumens in acute complicated type B aortic dissections after TEVAR with stent grafts alone or with a composite device design. J Vasc Surg. 2015;61:35S-36S.
-
Sobocinski J, Dias NV, Berger L, et al. Endograft repair of complicated acute type B aortic dissections. Eur J Vasc Endovasc Surg. 2013;45:468-474.
-
Barbante M, Sobocinski J, Maurel B, et al. Fenestrated endografting after bare metal dissection stent implantation. J Endovasc Ther. 2015;22:207-211.
-
Lee M, Lee do Y, Kim MD, et al. Outcomes of endovascular management for complicated chronic type B aortic dissection: effect of the extent of stent graft coverage and anatomic properties of aortic dissection. J Vasc Interv Radiol. 2013;24:1451-1460.
-
Sayer D, Bratby M, Brooks M, et al. Aortic morphology following endovascular repair of acute and chronic type B aortic dissection: implications for management. Eur J Vasc Endovasc Surg. 2008;36:522-529.
-
Canaud L, Faure EM, Ozdemir BA, et al. Systematic review of outcomes of combined proximal stent-grafting with distal bare stenting for management of aortic dissection. Ann Cardiothorac Surg. 2014;3:223-233.
-
Williams DM, Lee DY, Hamilton BH, et al. The dissected aorta: part III. Anatomy and radiologic diagnosis of branch-vessel compromise. Radiology. 1997;203:37-44.
-
Faure EM, Canaud L, Marty-Ane C, et al. Endovascular management of rupture in acute type B aortic dissections. Eur J Vasc Endovasc Surg. 2015;49:655-660.