Please click the button below and submit the required information to connect with your local Cook representative. This form is intended for EU-based physicians only.
The mini incubator that IVF clinics have
relied on for two decades just got better.

![]()
“…to reduce environmental stressors and to maintain appropriate growth conditions. This makes the laboratory incubator one of the most important pieces of equipment within the IVF laboratory and highlights the importance of incubator selection, which is one of the key decisions within the
IVF laboratory.”1
1. Swain JE. Decisions for the IVF laboratory: comparative analysis of embryo culture incubators. Reprod Biomed Online. 2014;28(5):535-547.
![]()
2. Mortimer D, Jansen R, Henman MJ. Development of an Improved Embryo Cultures System for Clinical Human IVF. Brisbane, Australia; Cook Australia; 2002.
![]()

Gas flow bubbles and water level can be observed at the front of the incubator.

• The chambers are 50% larger than in the previous generation of MINC.
• The two separate chambers can accept a total of 12 Nunc® 4-well dishes.
Nunc is a registered trademark of Nunc A/S Corporation.

• Premixed gas is supplied to each incubation chamber independently through a humidification flask.
• The MINC+ uses sterile, disposable humidification flasks to humidify and direct gas into each incubation chamber.
• Gas flow bubbles and humidification flask water levels are visible at the front of the device.

• MINC benchtop incubators have been an integral part of IVF practices worldwide for more
than 20 years.

• MINC+ continuously monitors critical functions.
• Alerts and status icons are visible on the front screens.

The DishTrace platform combines functionality through the incubator touchscreen and PC software to provide a range of dish data management tools.
DishTrace MINC+ allows you to access and log information via the touchscreen on the centre console.
• Add and edit dish records, assign dish locations, and view environmental settings.
• Check in and check out dishes.
See sample DishTrace MINC+ report
• DishTrace PC software allows you to monitor and manage up to 50 MINC+ incubators.
• View the current status and log the information for up to 50 MINC+ incubators connected to the local network.
• Add, edit, search, and view data records for registered dishes in the connected incubators.
• Generate and view graphical histories of the dishes, dish locations, and incubator alerts and conditions.
• Generate quality–control reports for all MINC+ activity as PDF or CSV files.
• View dish data that is entered through DishTrace MINC+.

• The media warming chamber is located between the incubator chambers, and is heated independently of the chambers.
• The MWC is not gassed and can provide a constant temperature for IVF-related liquids such as media and oil.
• The removable MWC cradle can hold up to six 17 mm test tubes.

• The MINC+ has a 12-year service life with a dedicated service and maintenance team.
• Comprehensive service plans and payment options are available in selected markets.
![]()
18 °C to 32 °C at temperature set point of 37.0 °C
20 °C to +28 °C for all other temperature settings
10% to 85% relative humidity (RH) 700 hPa to 1,060 hPa
MINC+ should be placed on a secure, level surface away from heaters, coolers,
air conditioning outlets, mists, splashes, and exposure to direct sunlight.
Door closed: 556 mm wide x 197 mm high x 449 mm deep
Door opened: 556 mm wide x 528 mm high x 449 mm deep
23.4 kg (51.6 lb)
It is advised that service personnel install the MINC+ Benchtop Incubator and routinely service the device.

Please refer to the product’s Instructions for Use (IFU) for full prescribing information, warnings, precautions, contraindications, and potential adverse events.
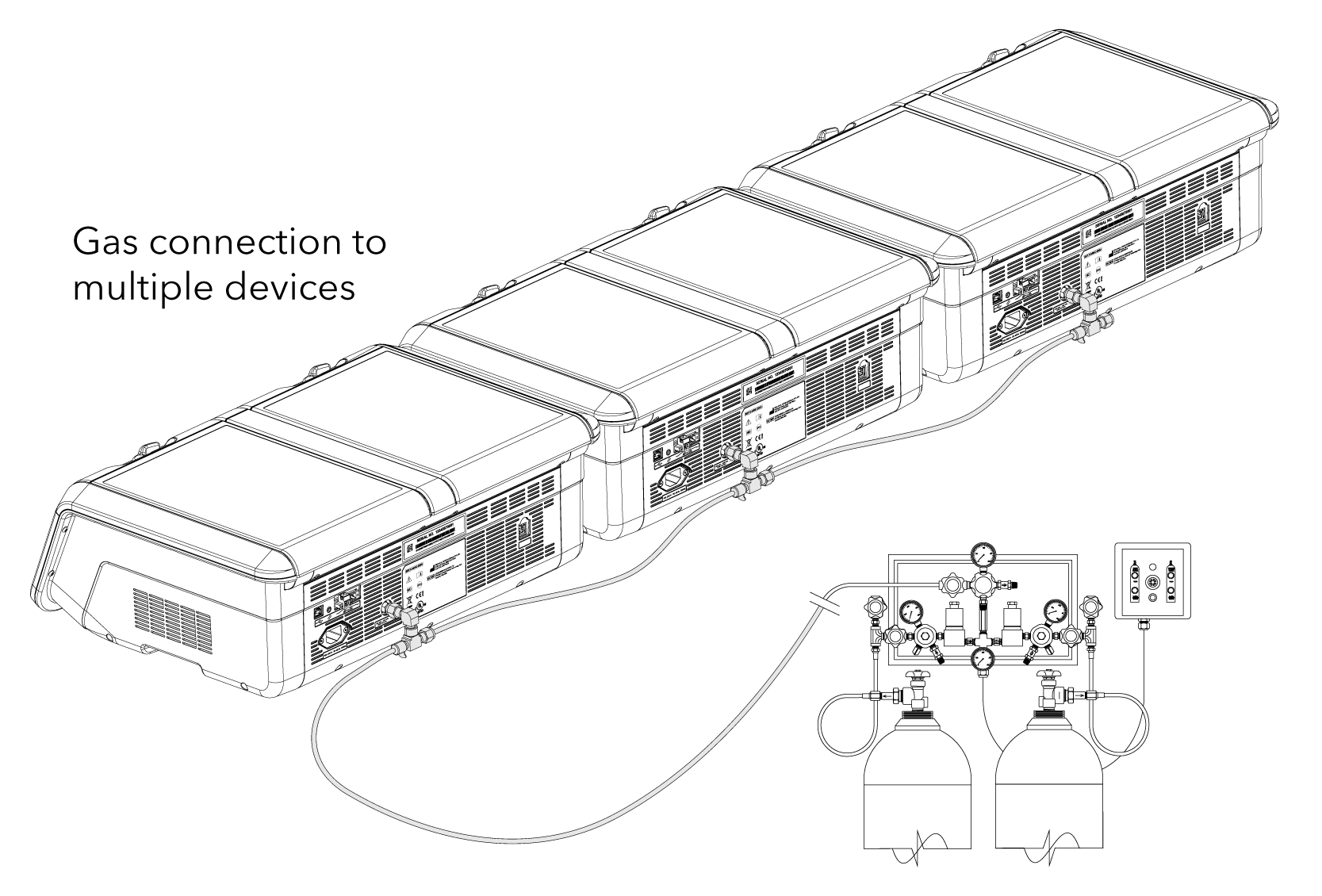
• Premixed gas required: CO2, O2, and N2. Concentrations will be dependent on the specification by laboratory personnel.
• Gas regulator: use only a medical-grade regulator set to a nominal 150 kPa.*
• Use the Braided Connecting Tube supplied with the MINC+.
• MINC+ can be connected as a single device or sequentially (up to 7 MINC+) from a single gas point.*
• 150 kPa ±15 kPa
– (21.8 psi ± 2.2 psi)
– (1,500 mbar ± 150 mbar)
• Blend of 6% CO2, 5% O2, 89% N2 (Cook culture system at sea-level) or high-purity 6% CO2 in air (recommended tolerances ± 0.2%)
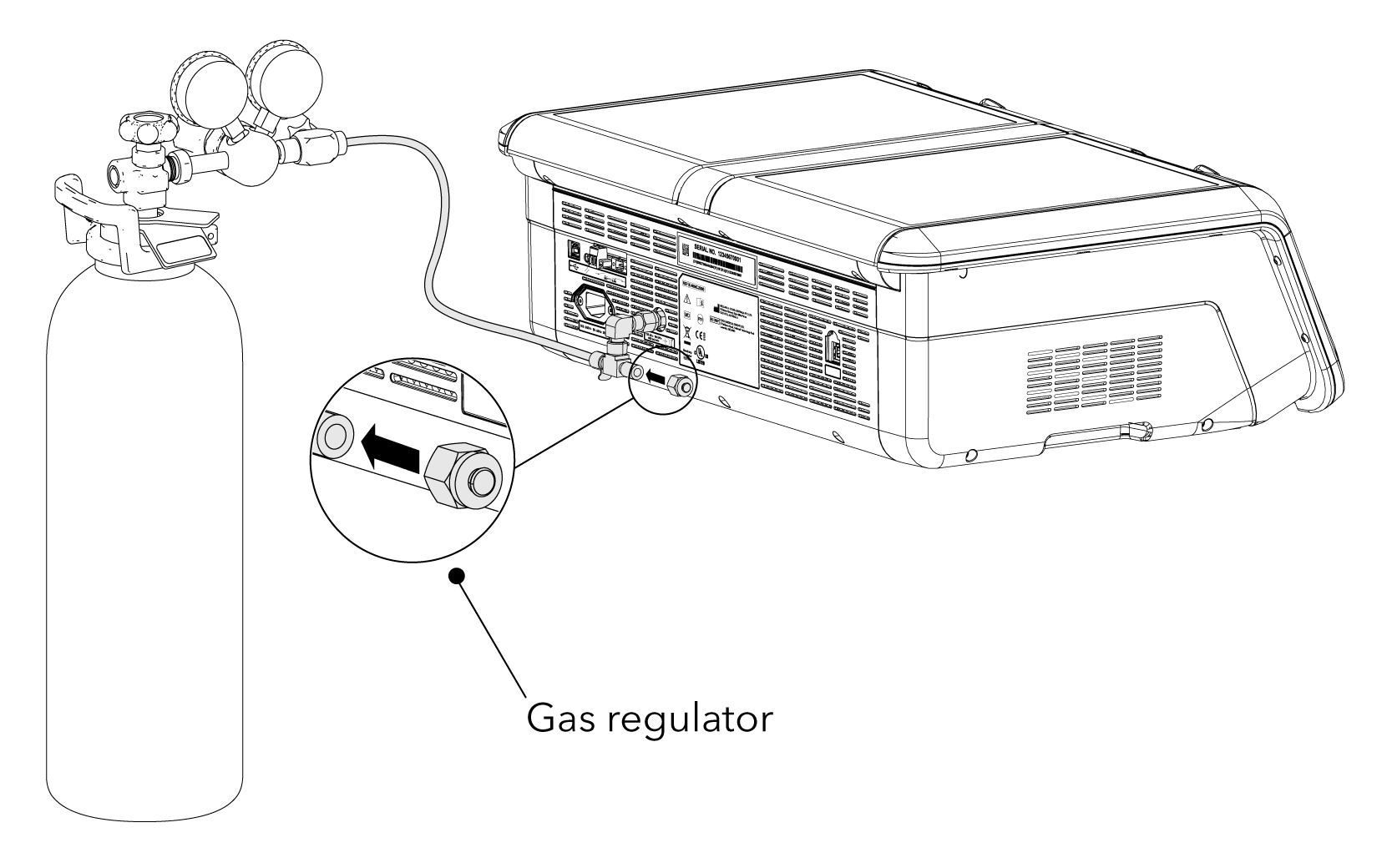
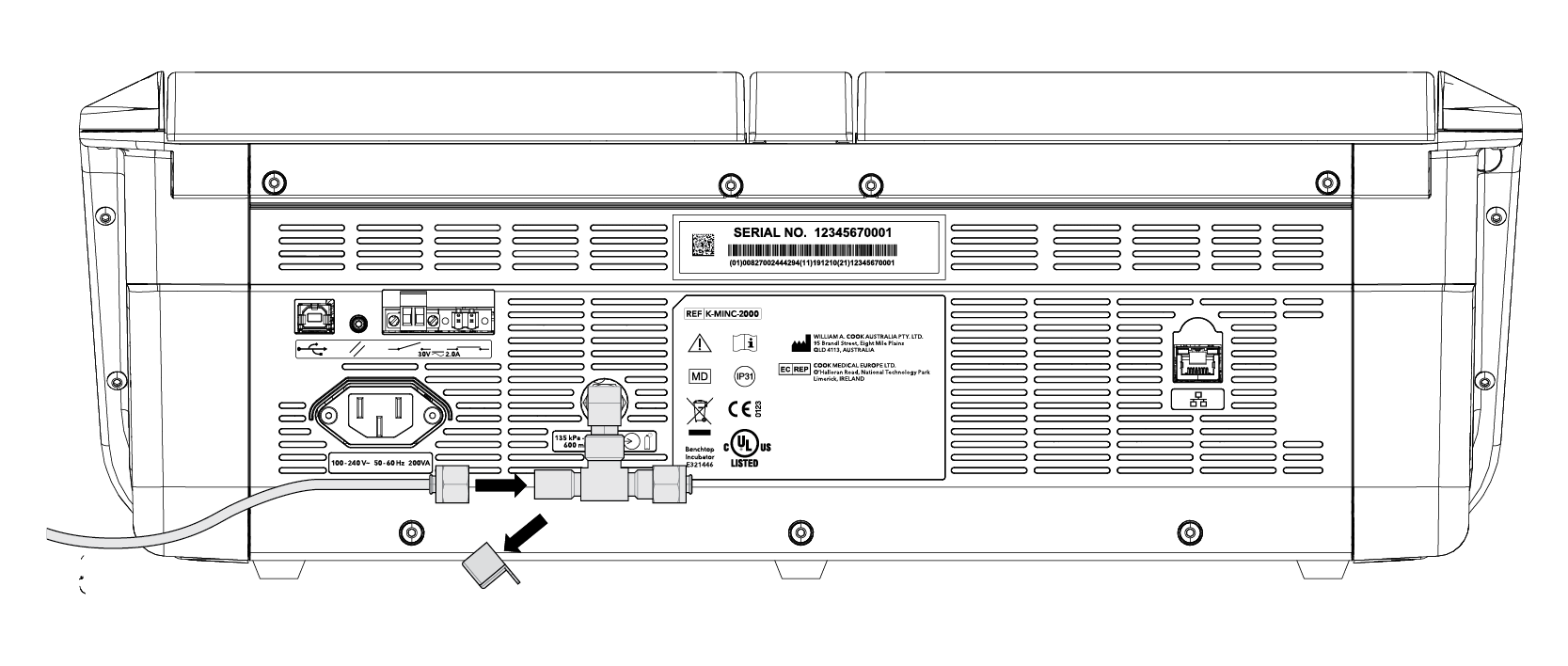
Back connection for a single device
*Please refer to the product’s Instructions for Use (IFU) for full prescribing information, warnings, precautions, contraindications, and potential adverse events.
The MINC+ can be connected to an external alarm system.
• It has two alarm configurations: normally open (NO) or normally closed (NC).
• It will activate under the following conditions:
– Loss of mains power
– Low inlet gas pressure
– Low water level in the humidification flasks
– No gas flow or gas flow out of range
– Temperature out of range
– Internal function error detected
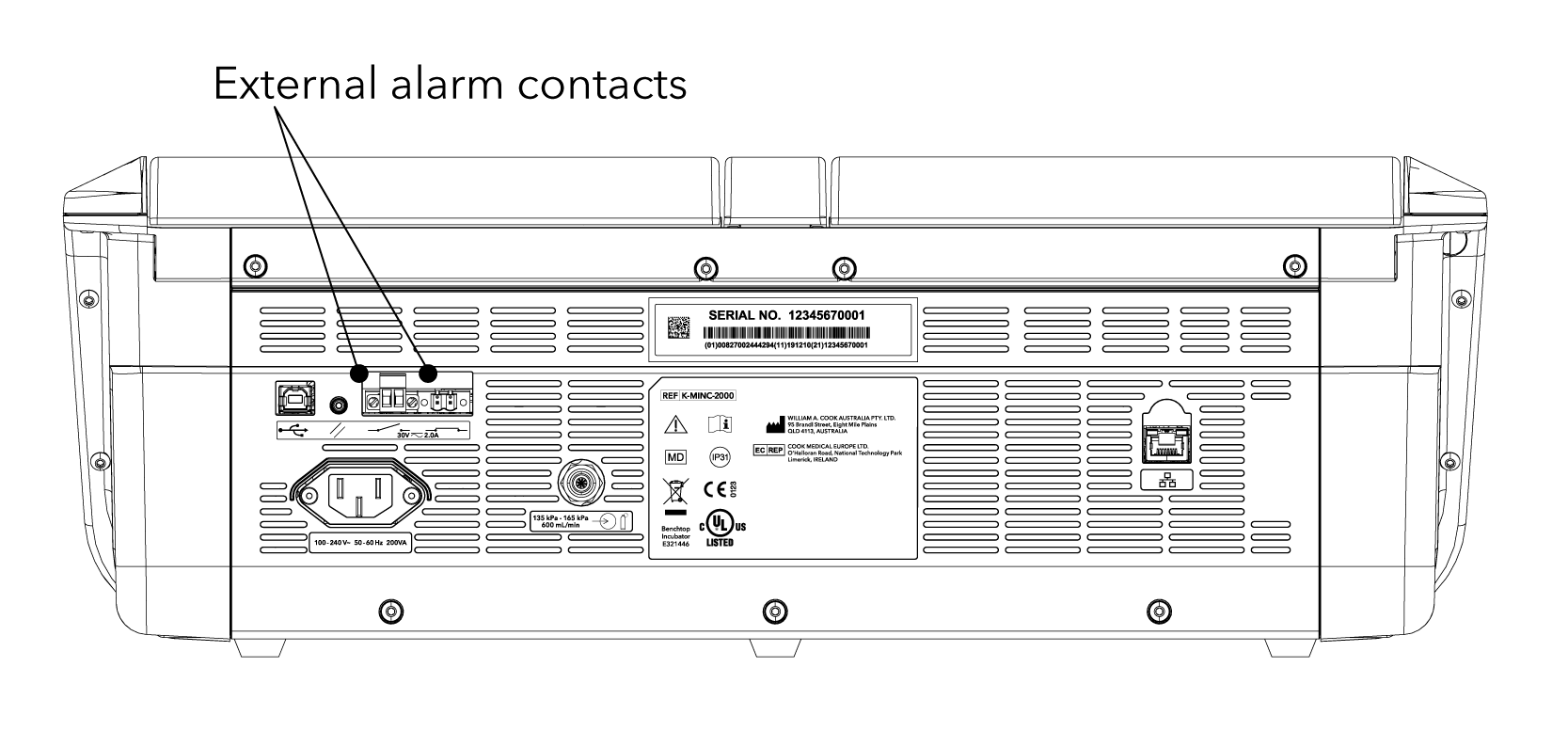
Please refer to the product’s Instructions for Use (IFU) for full prescribing information, warnings, precautions, contraindications, and potential adverse events.
![]()

+Toward a predictive theoretical model for osmolality rise with non-humidified incubation:
a randomized, multivariate response-surface study. (Mullen SF)
+Factors of the human embryo culture system that may affect media
evaporation and osmolality. (Mestres E, García-Jiménez M, Casals A, et al.)
+Impact of the type of incubator (non-humidified versus humidified) on embryo culture media osmolality. (Boumerdassi Y, Huet S, Millin M, et al.)
+Humidity and osmolality: Can we avoid media osmolality shifts in a dry environment? (Mullen SF)
+Controversies in ART: considerations and risks for uninterrupted embryo culture. (Swain JE)
+Unstable osmolality of microdrops cultured in non-humidified incubators. (Yumoto K, Iwata K, Sugishima M, et al.)
+Humidification of a dry benchtop IVF incubator: impact on culture media parameters. (Holmes R, Swain JE)
+Different mineral oils used for embryo culture microdrop overlay differentially impact media evaporation. (Swain JE)
+Media evaporation in a dry culture incubator; effect of dish, drop size and oil on media osmolality. (Swain JE, Graham C, Kile R, et al.)
+Media osmolality changes over 7 days following culture in a non-humidified benchtop incubator. (Swain JE, Schoolcraft WB, Bossert N, et al.)
+Unstable osmotic pressure in microdrops cultured under mineral oil in non-humidified incubators. (Iwata K, Yumoto K, Mio Y)
+Microdrop preparation factors influence culture-media osmolality, which can impair mouse
embryo preimplantation development. (Swain JE, Cabrera L, Xu X, et al.)
![]()
+Comparison of humidified versus non-humidified incubation with sequential culture media in
a time-lapse incubator using sibling oocytes splits. (Holmes R, Weinberg K, Kalaghan L, et al.)
+Humid vs. dry embryo culture conditions on embryo development: a continuous embryo
monitoring assessment. (Del Gallego R, Albert C, Marcos J, et al.)
+Humid versus dry incubator: a prospective, randomized, controlled trial. (Fawzy M, AbdelRahman MY, Zidan MH, et al.)
+Incorporation of the Cook K-MINC incubator and media system into the IVF lab: the future of IVF. (Lee M, Grazi R, Seifer DB)
+Cell volume regulation in oocytes and early embryos: connecting physiology to successful culture media. (Baltz JM, Tartia AP)
![]()

The product information on this website is intended only for physicians and healthcare professionals licensed in the European Union (except France), the United Kingdom, Switzerland, Norway, Iceland, Turkey, and Liechtenstein. If you are located in another global region, please click on the regional flag at the very top of the webpage and choose your region from the drop-down options.
If your region is not listed, visit our How to Order section for more information.
Information provided on this site is not intended to be professional medical advice. Product Instructions for Use (IFU) should be consulted before use of any product.